Question
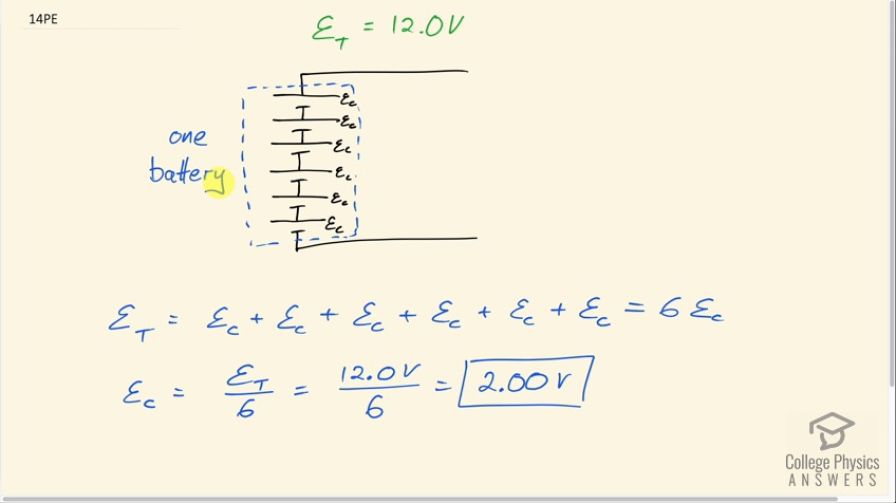
Standard automobile batteries have six lead-acid cells in series, creating a total emf of 12.0 V. What is the emf of an individual lead-acid cell?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 21, Problem 14 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Video Transcript
This is College Physics Answers with Shaun Dychko. Batteries always consist of many cells connected together in series and the meaning of the word 'battery' comes from you know... used to be that whole bunch of pieces of artillery that were firing together were considered a battery of artillery and now the word 'battery' can be used to describe a collection of whole bunch of things that are related... put together and it's in that sense that it applies to an electro-chemical battery in that you have many cells that are put together to work together as a battery. Okay! So if we traverse, you know, this part of a circuit, from this point and go to the top of the battery— from the bottom of the battery to the top— we cross one cell and then the next and then the next and then the next and then the next and the next until we have done that six times and each time, we are increasing that potential until we have reached the total potential difference, which is the total emf. And so the total emf then is the sum of all the emf's of each cell and there are six of them so if we are adding ε C to itself six times that means we can multiply ε C by 6 and then divide both sides by 6 to get the emf of a single cell. So that's 12.0 volts divided by 6 which is 2.00 volts. And each type of battery has its own characteristic potential difference for a single cell depending on its chemistry. So a lithium-ion battery would have a different voltage for a particular cell or a single cell and nickel–metal hydride again would be something else different as well.