Question
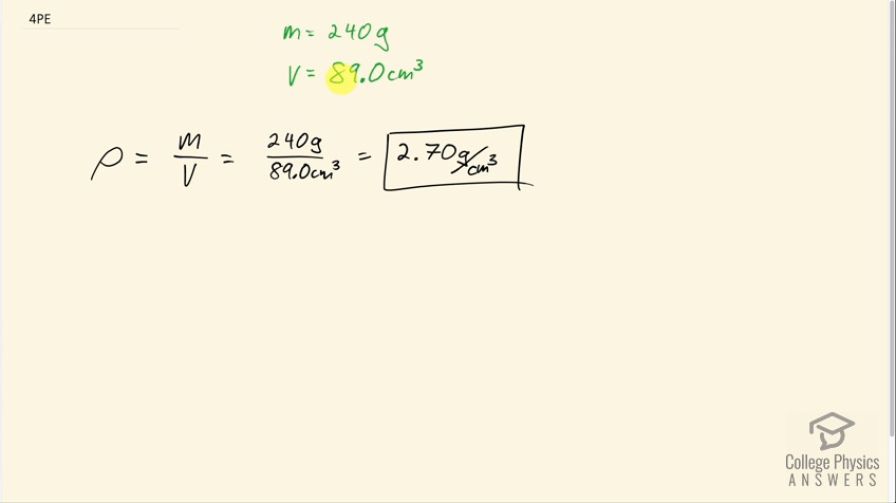
A straightforward method of finding the density of an object is to measure its mass and then measure its volume by submerging it in a graduated cylinder. What is the density of a 240-g rock that displaces of water? (Note that the accuracy and practical applications of this technique are more limited than a variety of others that are based on Archimedes’ principle.)
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 11, Problem 4 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. An object has a mass of 240 grams and a volume of 89.0 centimeters cubed and we have to find its density. So we divide the mass by the volume to find density so that's 240 grams divided by 89.0 cubic centimeters which is 2.70 grams per cubic centimeter.
