Question
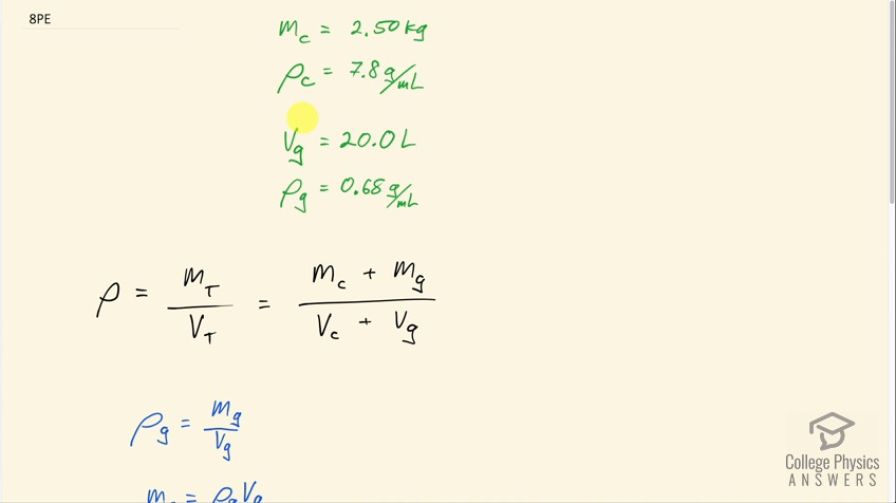
A 2.50-kg steel gasoline can holds 20.0 L of gasoline when full. What is the average density of the full gas can, taking into account the volume occupied by steel as well as by gasoline?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 11, Problem 8 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. A steel can with a mass of 2.50 kilograms is holding 20.0 liters of gasoline and we have to figure out what the average density of this can-gasoline combination is. So we need to know what the total mass of the can plus gasoline is and then divide that by the total volume of the can plus the gasoline. So we need to know the densities of the can material and the gasoline in order to figure out some of the unknowns here. We don't know the volume of the can like how much metal is used... like what volume of metal is used to make it and we don't know the mass of the gasoline either. So we look up in table [11.1] for the density of steel which is 7.8 grams per milliliter and the density of gasoline, which is petrol, is 0.68 grams per milliliter so that's where these numbers came from. So we need to find the mass of gasoline and so we know the density of gasoline is its mass divided by its volume and we are given the volume so we can use the density to figure out the mass. So we'll multiply both sides by the volume of gasoline here and we get that the mass of the gasoline is the density multiplied by the volume. Also we want to find the volume of steel used to make up the can so that's gonna be the mass of the steel divided by the density of steel. And so we make substitutions for these two unknowns: the mass of the gas is substituted with density of gasoline times volume of gasoline; and the volume of the can is substituted with mass of the can divided by the density of the can. So now when you are plugging in numbers, you have to be careful with units so the important thing is that you choose an unit system be it grams per milliliter or kilograms per cubic meter and pick one or the other and stick with those units throughout every number in your calculation. So I have chosen grams per milliliter and so whenever there's a mass, I need grams and whenever there's a volume, I need milliliters. So the mass of the can is 2.50 kilograms but the prefix 'kilo-' means times 10 to the 3 so I have written times 10 to the 3 grams and then add to that the 0.68 grams per milliliter—density of gasoline— multiplied by 20.0 liters but liters doesn't belong here—we want milliliters— so we multiply by 1000 milliliters for every liter. Then in the denominator, we have 2.50 times 10 to the 3 grams again divided by 7.8 grams per milliliter— density of steel— plus 20.0 liters but this has to be converted into milliliters and we end up with 0.79 grams per milliliter is the average density of the can-gasoline combination.
