Question
Calculate the gauge pressures inside 2.00-cm-radius bubbles of water, alcohol, and soapy water. Which liquid forms the most stable bubbles, neglecting any effects of evaporation?
Final Answer
The bubble formed by alcohol is the most stable since the gauge pressure within the bubble is lowest. In the real world which include evaporation, however, the alcohol bubble would quickly evaporate, in which case soapy water would create the longest lasting bubble.
Solution video
OpenStax College Physics for AP® Courses, Chapter 11, Problem 62 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
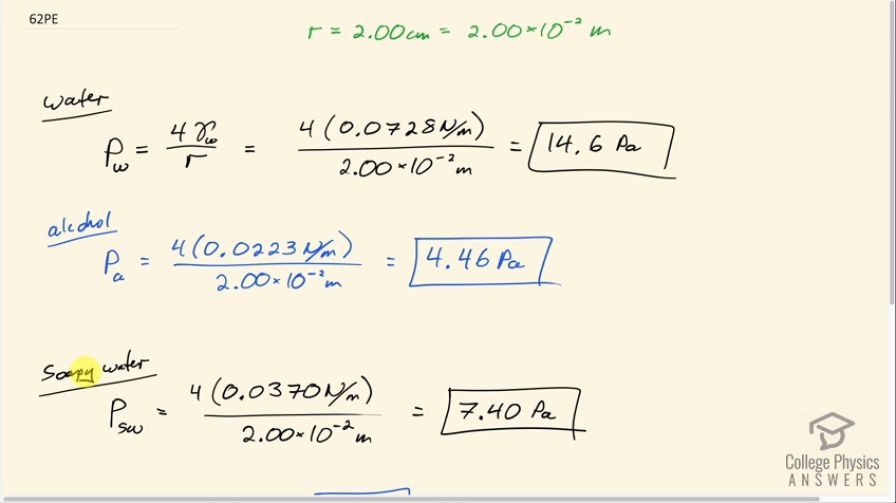
This is College Physics Answers with Shaun Dychko. We are going to calculate the gauge pressure inside bubbles made with different fluids—water, alcohol and soapy water— and the bubble has a radius of 2.00 centimeters and the prefix 'centa' means times 10 to the minus 2; we need to convert to meters in order to use in our formulas. So we have the gauge pressure when the bubble is made out of water is 4 times the surface tension of water which we look up in our data table and that is 0.0728 newtons per meter— we assume the water is at 20 degrees Celsius say— and this works out to 14.6 pascals after you divide by the radius of 2.00 times 10 to the minus 2 meters. And then for alcohol, it's gonna be 4 times 0.0223 newtons per meter which we look up here—we'll assume it's ethyl alcohol, 0.0223— divided by 2 times 10 to the minus 2 meters and that's 4.46 pascals and this gauge pressure is the amount by which the pressure inside the bubble exceeds atmospheric pressure and so it's 4.46 pascals more than atmospheric pressure. And the gauge pressure inside a bubble made of soapy water— we have to look up its surface tension— and that is 0.0370 and then this works out to 7.40 pascals. Now since the bubble formed by the alcohol has the lowest gauge pressure, it's going to be the most stable because you know, the pressure inside is not trying to explode the bubble it's very low pressure inside compared to outside. This is of course ignoring effects of evaporation and so in the real world, soapy water would in fact be the most stable bubble because alcohol would quickly evaporate and the bubble material would just disappear. So but anyway... the question told us to ignore evaporation so in that case the answer is alcohol would make the most stable bubble.
