Question
(a) A 75.0-kg man floats in freshwater with 3.00% of his volume above water when his lungs are empty, and 5.00% of his volume above water when his lungs are full. Calculate the volume of air he inhales—called his lung capacity—in liters. (b) Does this lung volume seem reasonable?
Final Answer
- This is a bit less than the volume of a milk jug, so yes, it is reasonable.
Solution video
OpenStax College Physics for AP® Courses, Chapter 11, Problem 53 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
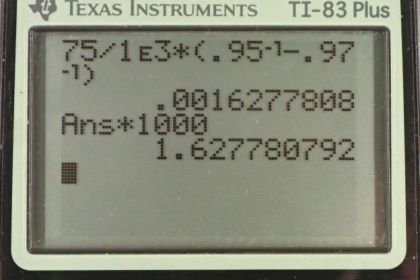
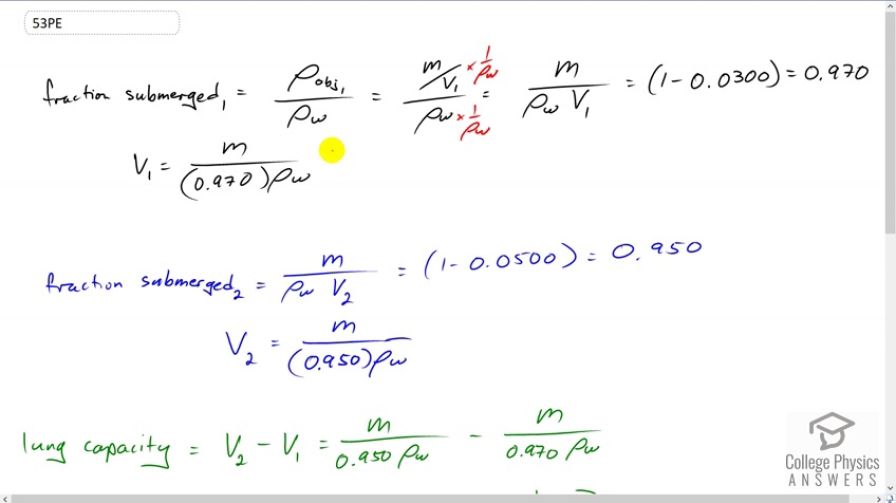
This is College Physics Answers with Shaun Dychko The textbook gives us the formula for the fraction of an object that is submerged and its the density of the object divided by the density of water. It has the subscript one for the first case when the man's lungs are empty, in which case the density of the person is m over the initial volume when the lungs are empty and divide that by density of water. Then I multiply top and bottom by one over rho w to get this fraction here. This equals one minus the three percent of the volume that is above the water. So that's 0.97 is the fraction submerged. We can solve this for V one by multiplying both sides by V one over 0.97 and you get this line here. So that's m over 0.97 times the density of water. Now in the second case we have the same expression here and it equals one minus 0.05 because five percent is above the water and that means the fraction submerged is 0.95. So V two is m over 0.95 rho w. Now the lung capacity is going to be the difference between these two volumes. So that's mass over 0.95 times rho w in the second case when the lungs are inflated, minus mass over 0.97 times rho w. This is the volume when the lungs are empty. We can factor out the m over rho w common factor there, times it by one over 0.95 minus one over 0.97. So it's 75 kilograms mass divided by one times ten to the three kilograms per cubic meter density of fresh water, times one over 0.95 minus one over 0.97 which gives this volume in cubic meters. Then we multiply it by 1000 liters for every cubic meter to get 1.63 liters is the volume of the man's lungs. That's a bit less than the volume of a milk jug and so yeah, it seems reasonable.