Question
A hydrogen atom in an excited state can be ionized with less energy than when it is in its ground state. What is for a hydrogen atom if 0.850 eV of energy can ionize it?
Final Answer
4
Solution video
OpenStax College Physics for AP® Courses, Chapter 30, Problem 12 (Problems & Exercises)
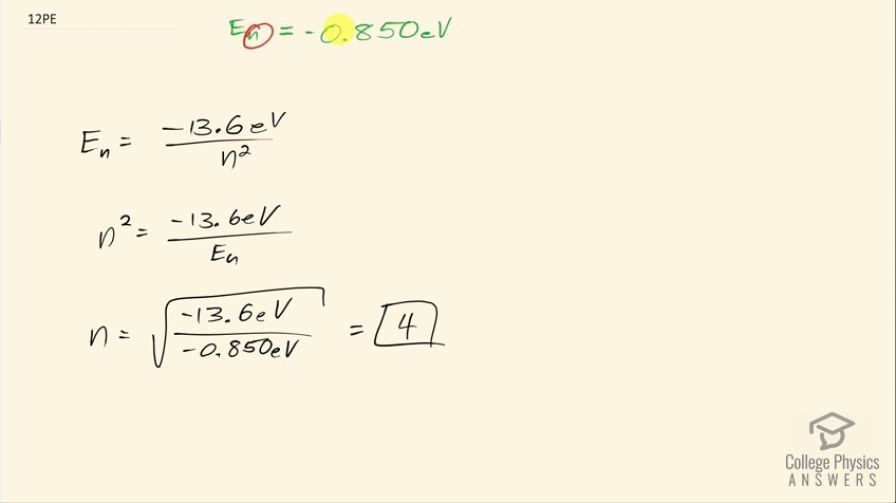
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. A hydrogen atom with an electron in some orbital n has an energy of negative 0.850 electron volts since this much energy is needed to ionize it and so when the electron is in the free ionized state— not really attached or bound to the atom— its energy is considered to be zero and this energy then is some amount below zero—that's why it's negative— and it's 0.850 below zero since 0.850 is needed... if energy has to be added to that electron in order to get it up to zero energy in its free unbound state. Okay! So the energy of the nth state then is negative 13.6 electron volts divided by the orbital number squared for hydrogen and we can solve this for n squared by multiplying both sides by n squared over the energy of the nth level and then take the square root of both sides and we get then that the orbital number then is the square root of negative 13.6 electron volts divided by negative 0.850 electron volts and the answer is 4 and we expected an integer and so this number is believable.
