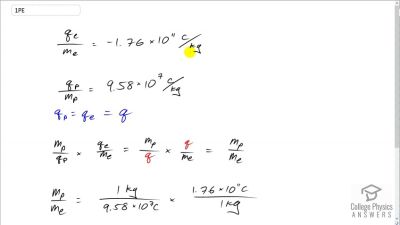
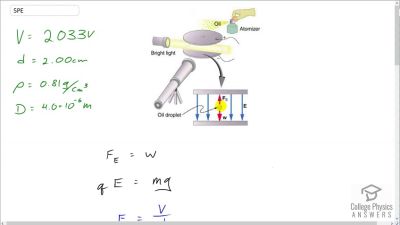
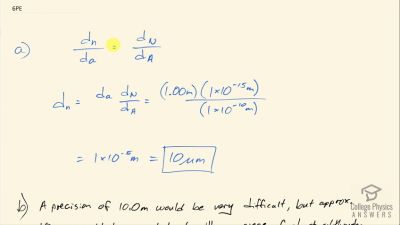
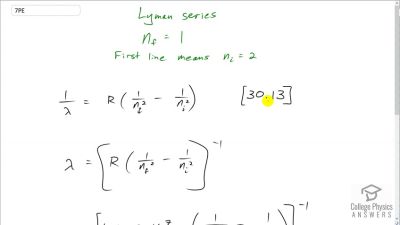
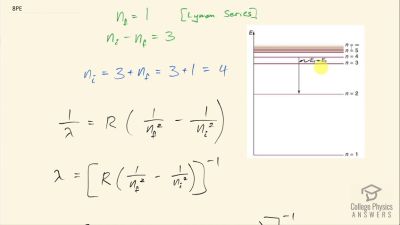
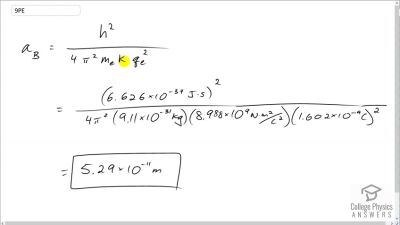
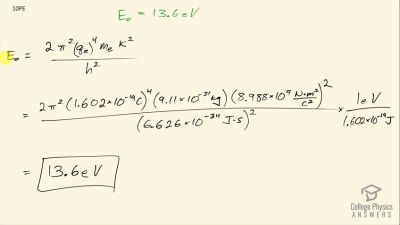
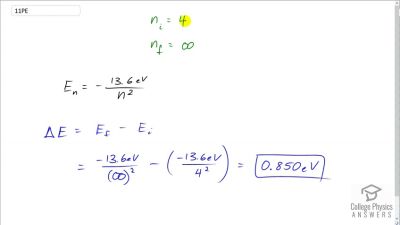
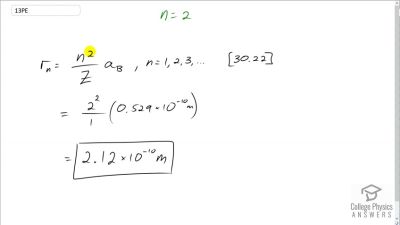
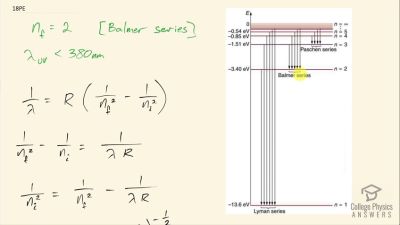
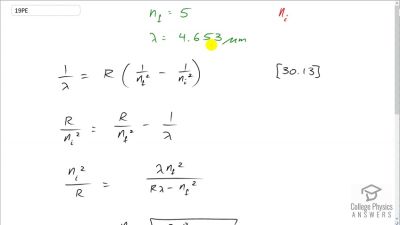
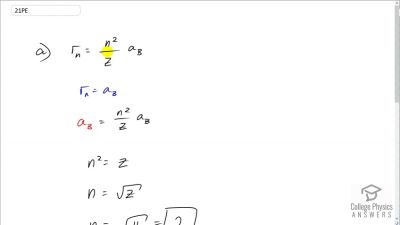
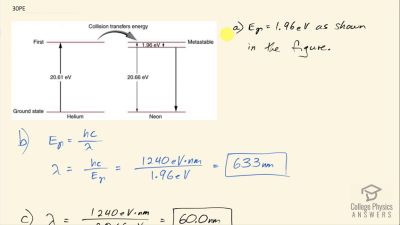
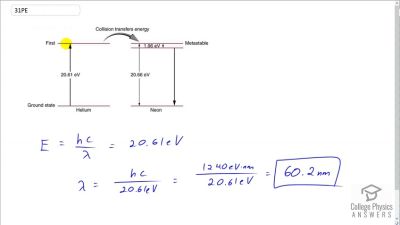
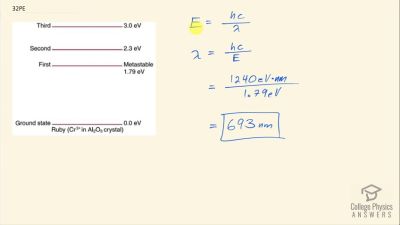
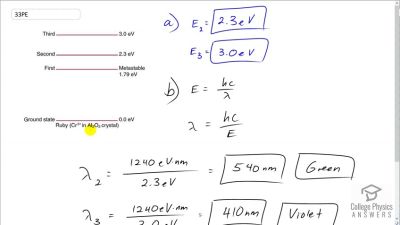
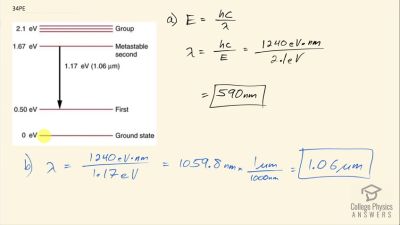
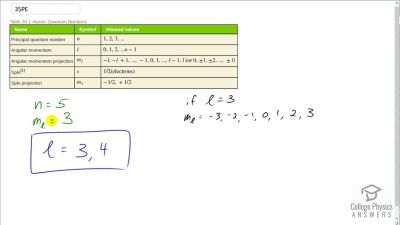
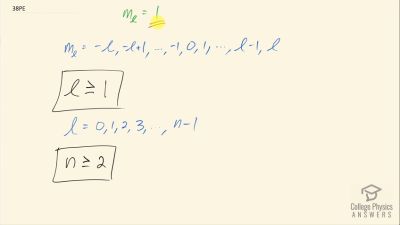
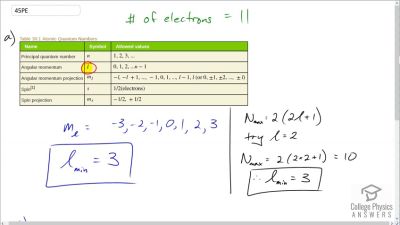
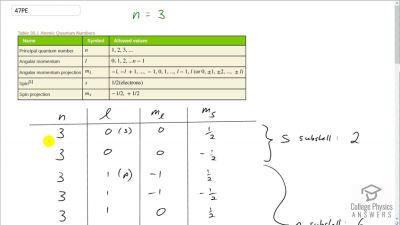
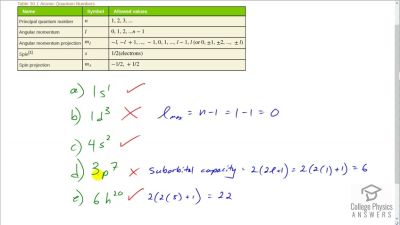
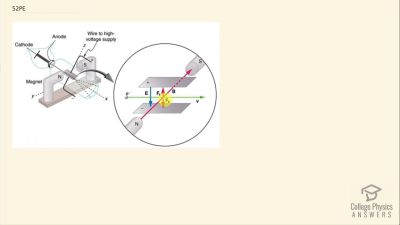
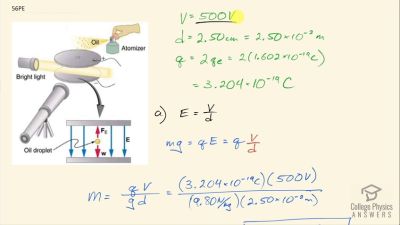
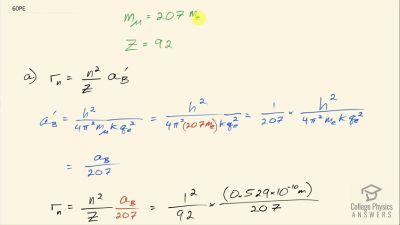
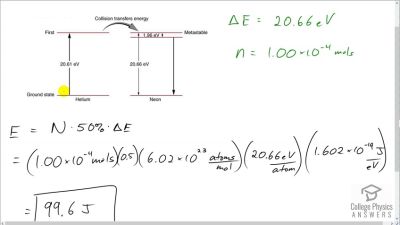
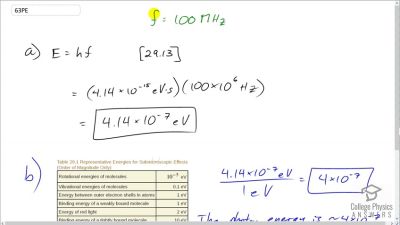
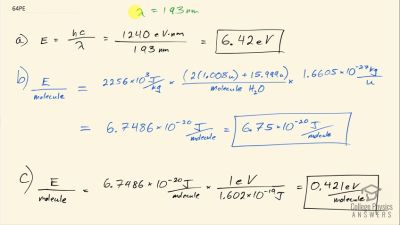
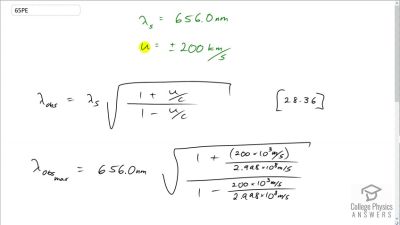
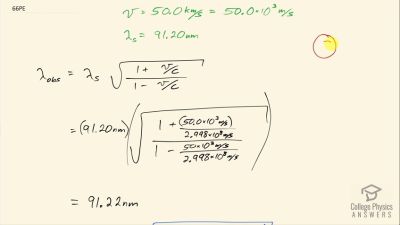
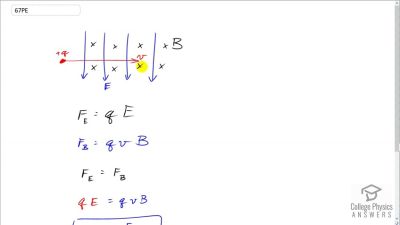
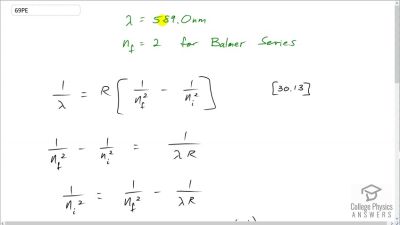
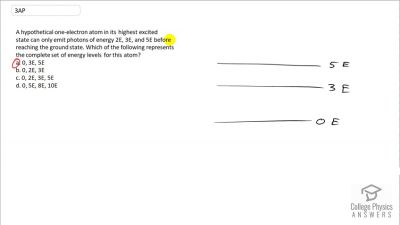
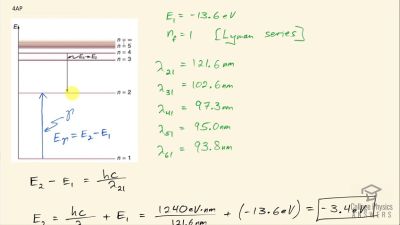
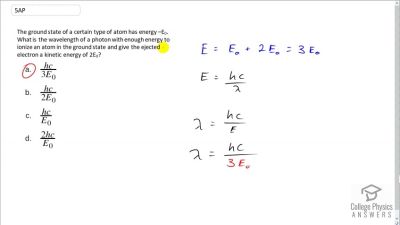
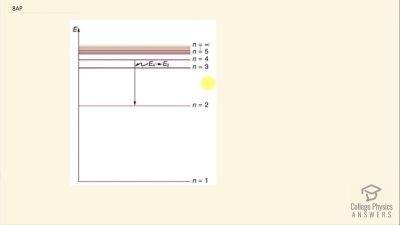
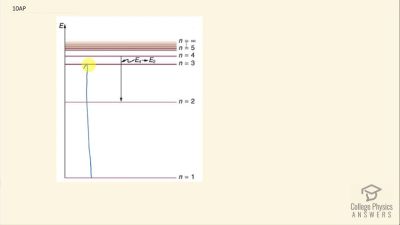
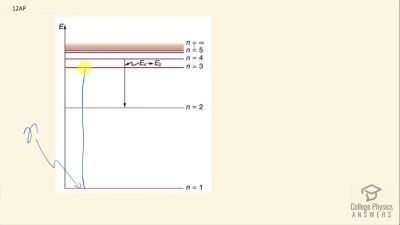
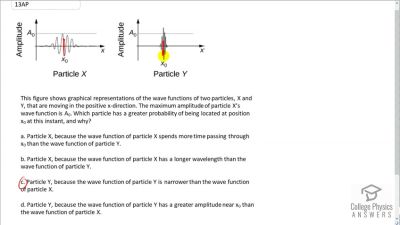
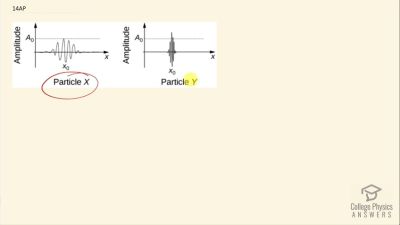
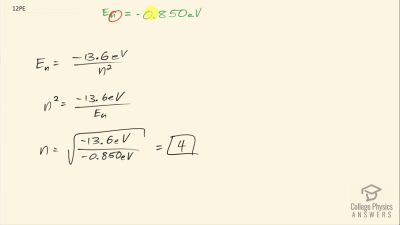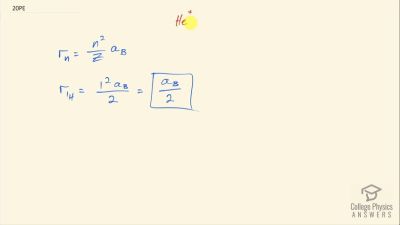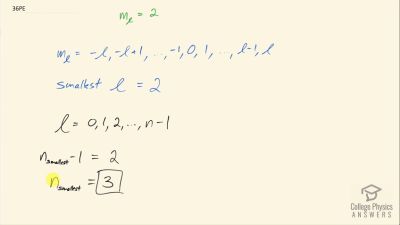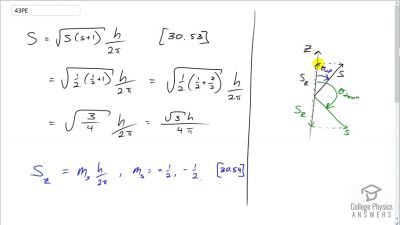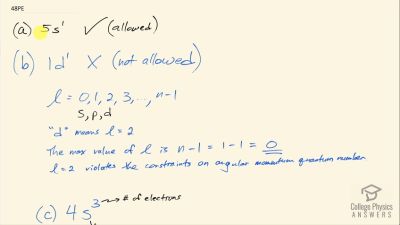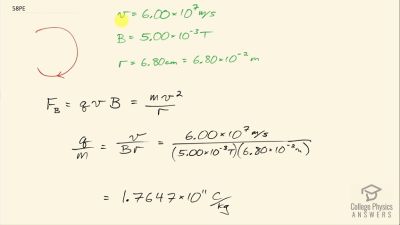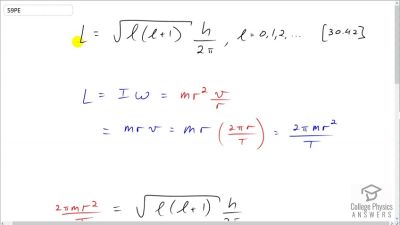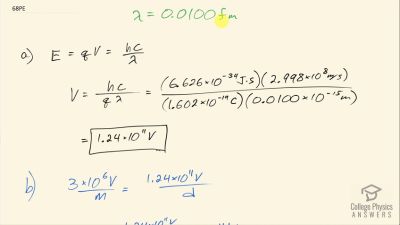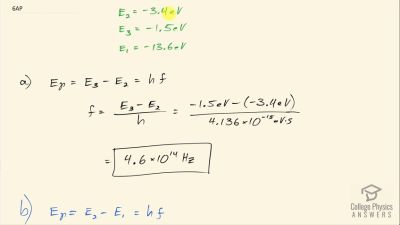In Millikan’s oil drop experiment, he experimented with various voltage differences between two plates to determine what voltage was necessary to hold a drop motionless. He deduced that the charge on the oil drop could be found by setting the gravitational force on the drop (pointing downward) equal to the electric force (pointing upward):
mdropg=qE ,
where
mdrop is the mass of the oil drop,
g is the gravitational
acceleration (
9.8 m/s2 ),
q is the net charge of the oil drop, and
E is the electric field between the plates. Millikan deduced that the charge on an electron,
e, is
1.6×10−19 C.
For a system of oil drops of equal mass (
1.0×10−15 kilograms), describe what value or values of the electric field would hold the drops motionless.