Question
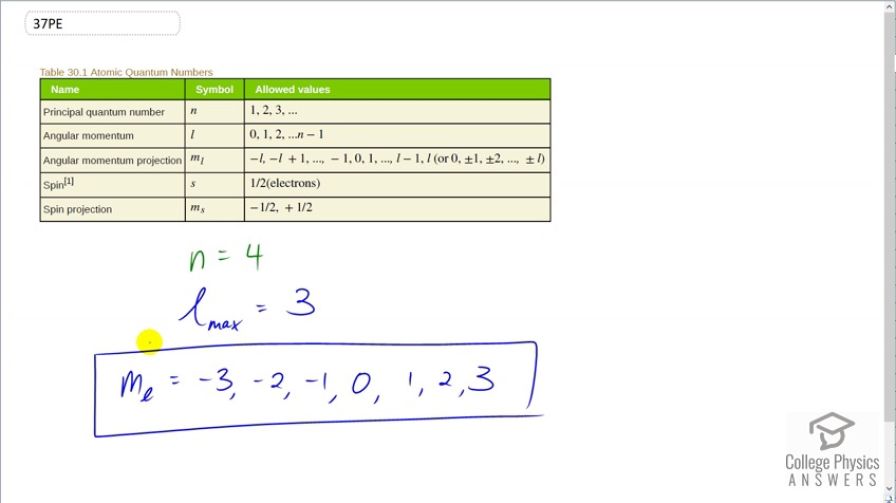
What are the possible values of for an electron in the state?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 30, Problem 37 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Video Transcript
This is College Physics Answers with Shaun Dychko. We are told that the principal quantum number for an electron around an atom is 4 and we are asked what are the possible values of the angular momentum projection quantum number, m l. Now, we don't know what l is because we are not told but we can choose a maximum l such that we get the maximum number of possibilities for m l because m l is possibilities are constrained by whatever the value of l is. And so the maximum possible l is 3 because it follows the sequence starting from 0, 1 to up to a maximum of principal quantum number minus 1 so that is 4 minus 1. So the maximum possible angular momentum quantum number is 3. So with that being 3, the angular momentum projection quantum number can start from negative 3 which is negative l and increasing by 1's to negative 2, negative 1, 0, 1, 2 or a maximum of l so a maximum of 3. So these are the possible values for m l given a principal quantum number of 4.