Question
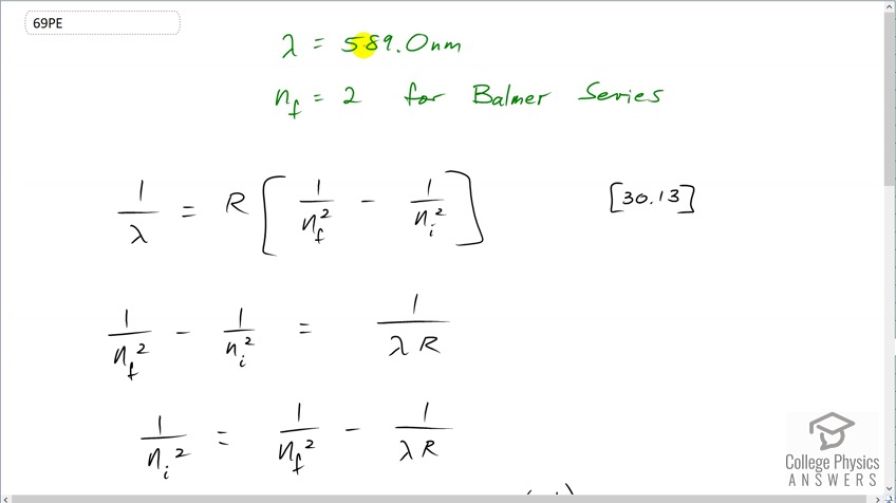
A student in a physics laboratory observes a hydrogen spectrum with a diffraction grating for the purpose of measuring the wavelengths of the emitted radiation. In the spectrum, she observes a yellow line and finds its wavelength to be 589 nm. (a) Assuming this is part of the Balmer series, determine , the principal quantum number of the initial state. (b) What is unreasonable about this result? (c) Which assumptions are unreasonable or inconsistent?
Final Answer
- This is not an integer.
- Either the wavelength measurement is not correct, or this is not a Balmer Series emission, or the gas is not hydrogen.
Solution video
OpenStax College Physics for AP® Courses, Chapter 30, Problem 69 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. A student observes an emission from a hydrogen atom of 589.0 nanometers and assumes that this is an emission from the Balmer series which means that n f is 2 in this formula [30.13]. And we are gonna figure out what is n i, what was the initial energy level for this electron? So we have to solve for n i and we are gonna divide both sides by Rydberg's constant and that means 1 over n f squared minus 1 over n i squared is 1 over λ times R and then we'll move this term to the right-hand side and move this term to the left-hand side and we are left with 1 over n i squared after we switch the sides around is 1 over n f squared minus 1 over λR. And then we can raise both sides to the exponent negative to flip this fraction and then make it one-half in order to take the square root so the exponent one-half is the same as writing a square root sign and the negative flips the fraction leaving us with n i on the left. And then what we do to the left, we also have to do to the right and so we wrap that in brackets and put exponent negative one-half and we'll leave it written that way. So the initial energy level then is 1 over 2 squared minus 1 over the observed wavelength— 589 times 10 to the minus 9 meters— times Rydberg's constant all to the power of negative one-half which is 3.24. And that is not reasonable because it's not an integer and i has to be an integer. So either the wavelength measurement is not correct or this is not a Balmer series emission and so n f is not 2 or this gas is not hydrogen at all.
