Question
What, if any, constraints does a value of place on the other quantum numbers for an electron in an atom?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 30, Problem 38 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Video Transcript
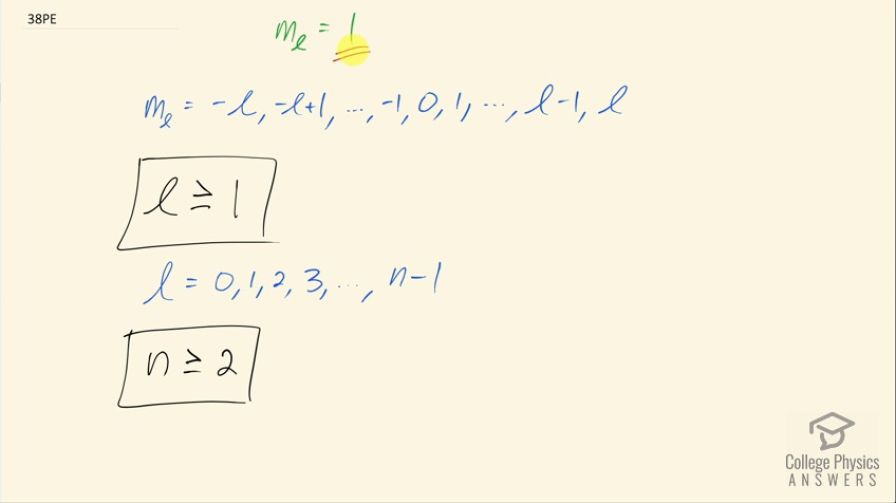
This is College Physics Answers with Shaun Dychko. We are told that the angular momentum projection number is 1 and we are asked what constraints does this value place on the other quantum numbers? So the formula or the sequence of possible values for the angular momentum projection number is negative l up to l in steps of integers. So with m l being 1, l cannot be zero because the maximum possible value for the projection number is l itself and so given that this angular momentum projection number is 1 l cannot be less than that. So l can be anything greater than or equal to 1; that's the constraint placed on the angular momentum quantum number. And then we can look at this sequence of possible values for the angular momentum quantum number to figure out constraints on principal quantum number n. So given that l is at least 1, what constraint does that place on n? Well the maximum possible value for l is 1 less than n so given that l is at least 1 that makes n greater than or equal to 2 because you know the smallest possible value for n is going to be 2 since 2 minus 1 gives this value but n could be any number bigger than that as well. So there we go!