Question
Describe the process of fluorescence in terms of the emission of photons as electron transitions between energy states. Specifically, explain how this process differs from ordinary atomic emission.
Final Answer
Please see the solution video.
Solution video
OpenStax College Physics for AP® Courses, Chapter 30, Problem 12 (Test Prep for AP® Courses)
vote with a rating of
votes with an average rating of
.
Video Transcript
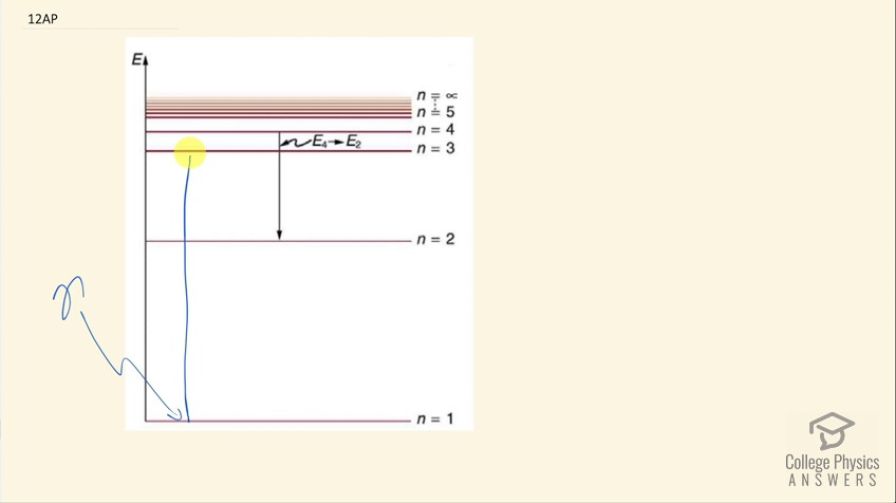
This is College Physics Answers with Shaun Dychko. Ordinarily when a photon hits a ground state electron and excites it up to some higher energy level, the electron will immediately return back to the ground state in one step and so whatever energy this photon had that caused the excitation that's the same energy of photon that will be re-emitted when the electron transitions back to the ground state. Now what makes phosphorescence different from this typical atomic emission is that the re-emission occurs in steps so instead of transitioning back to n equals 1 right away, the electron will go from n equals 3 to n equals 2 suppose— I am just using these numbers as examples— but the concept is that there's a step-wise return to ground level and in each step, a different wavelength photon is emitted so whereas originally... maybe these colors that I am using are actually instructive so blue could be a high energy photon that's incident causing this original excitation and then afterwards well, this one should be red because it's low energy because it's a short step here maybe you will have a red photon emitted during the transition from 3 to 2 and then it will go the final step from 2 to 1— I am drawing that in green because green is more energetic than red but less so than blue— and you will get two photons emitted— one for each transitions— and each of these photons has less energy than the original incident photon that caused the original excitation; this is fluorescence that I am describing here and there we go!