Question
(a) What is the magnitude of the angular momentum for an electron? (b) Calculate the magnitude of the electron’s spin angular momentum. (c) What is the ratio of these angular momenta?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 30, Problem 41 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
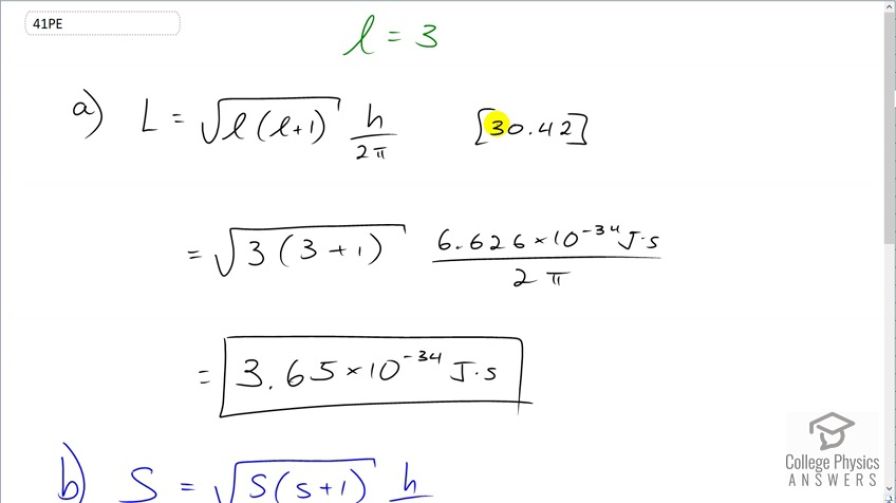
This is College Physics Answers with Shaun Dychko. We are going to calculate the magnitude of the angular momentum given an angular momentum quantum number 3 and so we have this formula [30.42] which says it is square root of l times l plus 1 times Planck's constant over 2π. So we have a square root of 3 times 3 plus 1 times 6.626 times 10 to the minus 34 joule seconds divided by 2π which is 3.65 times 10 to the minus 34 joule seconds. And we are gonna compare this angular momentum to the spin angular momentum. Now, for an electron the spin quantum number is always one-half and so this spin angular momentum is going to be square root of a half times a half plus 1 times h over 2π. This works out to 9.13 times 10 to the minus 35 joule seconds. And part (c) says find the ratio of the angular momentum divided by the spin angular momentum. And that is these two answers divided by each other and that's about 4.00. So the angular momentum is 4 times that of the spin angular momentum. And I suppose, you could call this the orbital angular momentum divided by the spin angular momentum.

