Question
Explain what phosphorescence is and how it differs from fluorescence. Which process typically takes longer and why?
Final Answer
Please see the solution video.
Solution video
OpenStax College Physics for AP® Courses, Chapter 30, Problem 10 (Test Prep for AP® Courses)
vote with a rating of
votes with an average rating of
.
Video Transcript
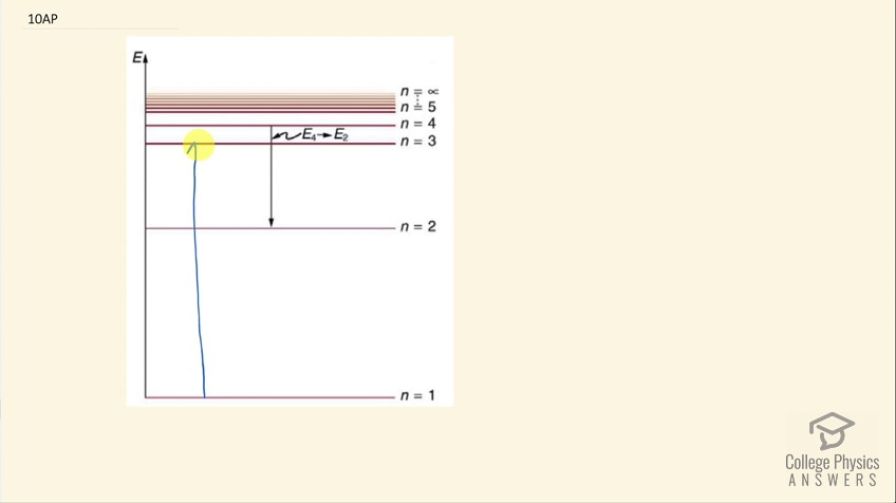
This is College Physics Answers with Shaun Dychko. With fluorescence, you have a high energy photon exciting an electron up to some high energy state and then the electron will transition back to the ground state in steps and in each step, it will emit a photon with less energy than the incident photon that originally excited the electron whereas this one might be an ultraviolet photon that caused this large excitation up to n equals 3— the photons emitted in these steps will have lower energy and could therefore be a longer wavelength and visible— and this fluorescence happens really quickly so the electron immediately descends back down to its ground state emitting these visible photons and there's no delay. But when it comes to phosphorescence, in that case the state that the electron was excited to is a metastable state and for some reason, the electron will stay in this n equals 3 state— suppose it's n equals 3— the electron will stay there for a while and then eventually later transition back down to the ground state probably in steps like this again so that the emitted photons are visible and this could take hours like in, you know, star stickers that are put on the wall that glow in the dark at night those are examples of phosphorescent stickers that have electrons excited to this metastable state and it will stay there for hours and then one by one gradually step back down to the ground state releasing visible photons in the process. So the difference between phosphorescence and fluorescence is, for the most part, the time scale. So fluorescence causes re-emission immediately whereas phosphorescence, the electrons stay in this metastable state for a while before transitioning back to the ground state.