Question
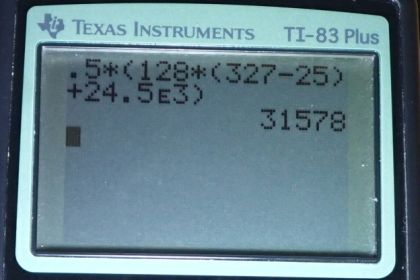
Some gun fanciers make their own bullets, which involves melting and casting the lead slugs. How much heat transfer is needed to raise the temperature and melt 0.500 kg of lead, starting from ?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 14, Problem 29 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko In this question, we’re going to melt half a kilogram of lead in order to cast it into a bullet. And so, we need to raise the temperature of the lead from its initial temperature of 25 degrees Celsius up to the melting point of lead, which is 327 degrees Celsius. And then, that will be given by this term that much heat, mass, times specific heat of lead, times the change in temperature. And then after getting to that temperature, we need additional heat to change the phase from solid into liquid. So that would be mass times the specific heat of fusion of lead. And I’ve written down the information we need up here: specific heat of lead, and the latent heat of fusion here. Okay, so, we can factor the mass out, which is a common factor from both of these terms. Then we have mass, times specific heat of lead, times change in temperature, plus the heat of fusion. And then that’s half a kilogram, times 128 Joules per kilogram per Celsius degree (specific heat of lead), times 327 degrees Celsius minus 25 degrees Celsius, plus the heat of – the latent heat of fusion 24 and a half times 10 to the three Joules per kilogram. We end up with 3.16 times 10 to the 4 Joules of energy, are needed to heat up and then melt the lead.