Question
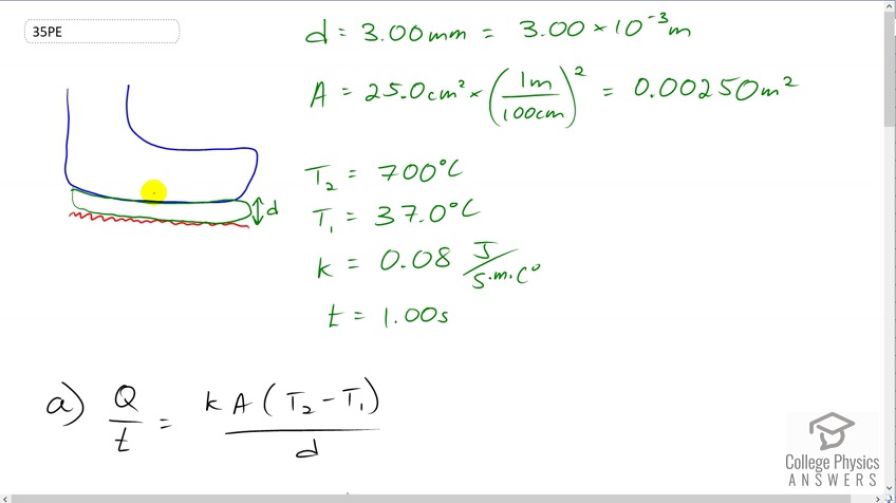
- A firewalker runs across a bed of hot coals without sustaining burns. Calculate the heat transferred by conduction into the sole of one foot of a firewalker given that the bottom of the foot is a 3.00-mm-thick callus with a conductivity at the low end of the range for wood and its density is . The area of contact is , the temperature of the coals is , and the time in contact is 1.00 s.
- What temperature increase is produced in the of tissue affected?
- What effect do you think this will have on the tissue, keeping in mind that a callus is made of dead cells?
Final Answer
- The callus cells are expendable since they're dead cells. The living tissue of the foot can easily sustain a rise in temperature without damage.
Solution video
OpenStax College Physics for AP® Courses, Chapter 14, Problem 35 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. A fire walker with a callus on a bottom of their foot with thickness of 3 millimeters which I have drawn in green here is walking on a bed of coals which has a temperature T2 of 700 degrees Celsius and we want to know how much thermal energy is transferred to the foot assuming that they step on the coals for 1 second and the temperature inside the foot is body temperature and that is 37 degree Celsius and so we have a formula for the rate of heat transfer due to conduction and we multiply both sides by t to solve for Q which is the amount of energy that will be transferred and other information we are given is that the area of foot is 25 squares centimeters which we multiply by 1 meter for every 100 centimeters and we multiply that twice and that’s gonna make 0.0025 square meters and the thermal conductivity of tissue is 0.08 Joules per second per meter per Celsius degree. So multiply both sides by t there to solve for Q and we end up with this formula and so we multiply the thermal conductivity 0.08 Joules per second per meter per Celsius degree times the area 0.0025 square meters times the temperature difference 700 degree Celsius minus 37 degree Celsius multiplied by the time of 1 second divided by 3 millimeters and this gives 40 Joules of energy transferred. Now part B says what will the temperature increase of the foot tissue be, given that it receives this much energy. Well, we know the density is 300 kilograms per cubic meter and the volume of tissue is 25 cubic centimeters and we multiply that by 1 meter for every 100 centimeters three times to give 2.5 times 10 to the minus 5 cubic meters and the specific heat of tissue is three thousand five hundred Joules per kilogram per Celsius degree so we need to use this formula to figure out what delta T will be and we divide both sides by mc and then switch the sides around and we get the change of temperature is the amount of heat energy absorbed divided by mass divided by specific heat. Now mass we need to figure out using density. So density is defined as mass per volume and so multiply both sides by V here and then switch the sides around to solve for m. So m is density times volume. And we will substitute that in place of m and we've written that down here in red. So, change of temperature is Q over ρVC that’s 44.2 Joules divided by 300 kilograms per cubic meter times 2 and a half times 10 to the minus 5 cubic meters times three thousand five hundred Joules per kilogram per Celsius degree which is 2 Celsius degrees temperature increase. Now the tissue of the foot can easily sustain a 2 Celsius degree rise in temperature without damage and you know, the cells at the very bottom of the callus that are directly touching the coals might get damaged because they have a higher temperature but they are expendable since they are dead cells so there will probably be no problem for the fire walker with such a quick step of only one second despite the fact that the coals are 700 degree Celsius. If they were to put their foot on the coals for much longer then there will be a different story.

