Question
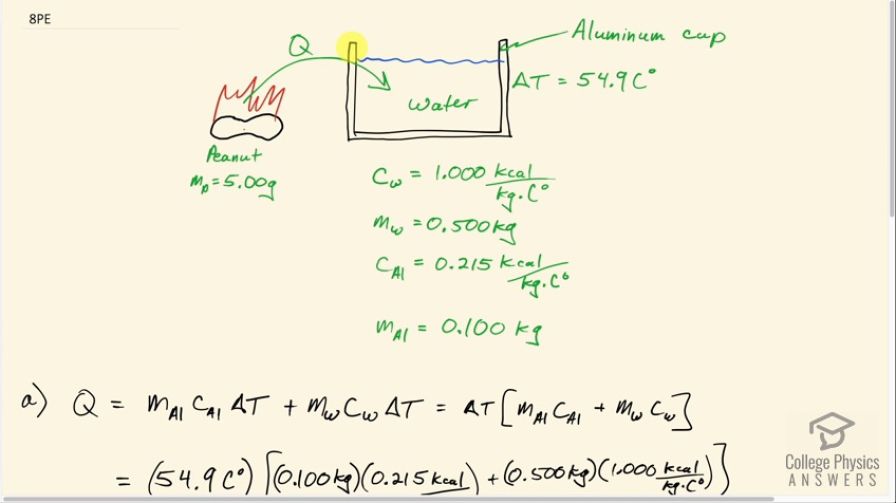
(a) The number of kilocalories in food is determined by calorimetry techniques in which the food is burned and the amount of heat transfer is measured. How many kilocalories per gram are there in a 5.00-g peanut if the energy from burning it is transferred to 0.500 kg of water held in a 0.100-kg aluminum cup, causing a temperature increase? (b) Compare your answer to labeling information found on a package of peanuts and comment on whether the values are consistent.
Final Answer
-
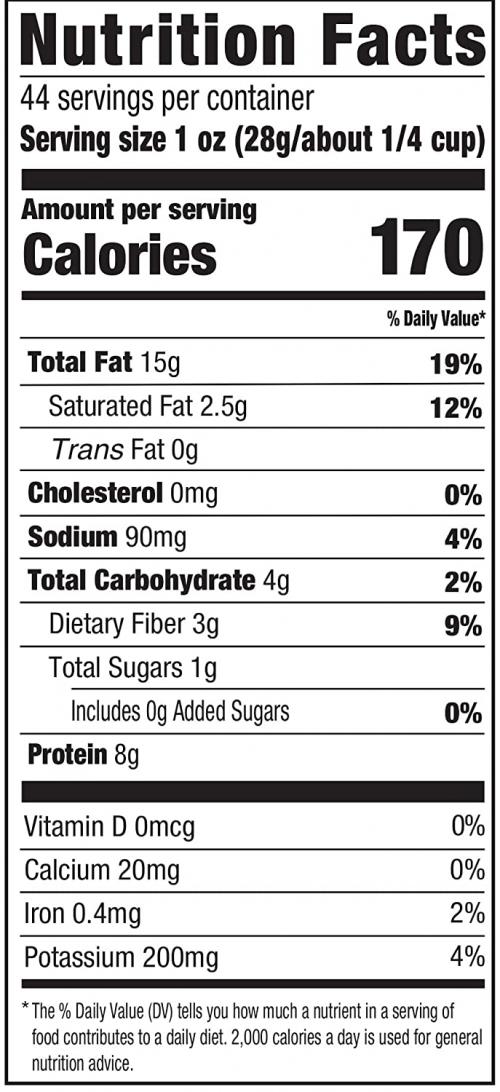
Note: at the end of the calculation for part (a) in the video I mistakenly wrote the units for the final answer as but it should be per gram. The units for the final answer to part (a) should be . - According to the peanuts nutrition label in Figure 14.a above, peanuts have . This agrees with our calculation to within 1 significant figure.
Solution video
OpenStax College Physics for AP® Courses, Chapter 14, Problem 8 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
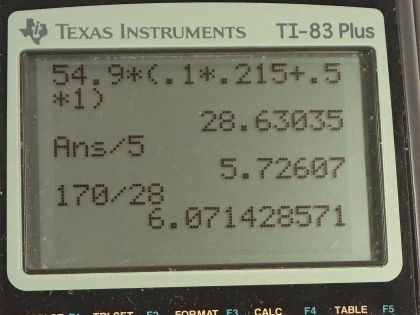
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. This peanut is burned and all of the heat energy is captured in this water and the aluminum container holding the water and based on knowing how much heat energy this is, we'll say that that's the total energy in this peanut and we'll figure out how much energy it has per gram in units of food calorie's per gram or kilocalories per gram. So there's a 0.100 kilogram aluminum cup holding the water and the aluminum has a specific heat of 0.215 kilocalories per kilogram per Celsius degree— that's what we look up in table [14.1]— and there is 0.500 kilograms of water which has a specific heat of 1 kilocalorie per kilogram per Celsius degree and the change in temperature of both the water and the aluminum cup together is 54.9 Celsius degrees. So the total amount of energy that's added to this water and aluminum combination is the mass of aluminum times the specific heat times the change in temperature plus the mass of the water times the water's specific heat times its change in temperature but the change in temperature is the same for both substances and so it doesn't need a subscript and it can be factored out. So we have ΔT times the mass and specific heat of the aluminum and water, respectively. So that's 54.9 Celsius degrees— change in temperature— times 0.100 kilograms of aluminum times 0.215—specific heat— plus 0.500 kilograms of water times 1.000 kilocalorie per kilogram per Celsius degree and we get 28.6304 kcal of energy. Now to figure out how many kilocalories there are per gram, we can take that amount of heat energy and divide it by 5.00 grams of the peanut and then that's 5.73 kilocalories per kilogram. Now on a peanut nutrition label that I found on Amazon, it says there are 170 Calories with a 'C' in every 28 grams and calories with a capital 'C' is the same as kilocalories. So 170 divided by 28 is 6.1 kilocalories per gram and yes, to one significant figure, this would be 6 if you rounded it and this is 6 if you rounded it to the ones place... these numbers agree and there we go!