Question
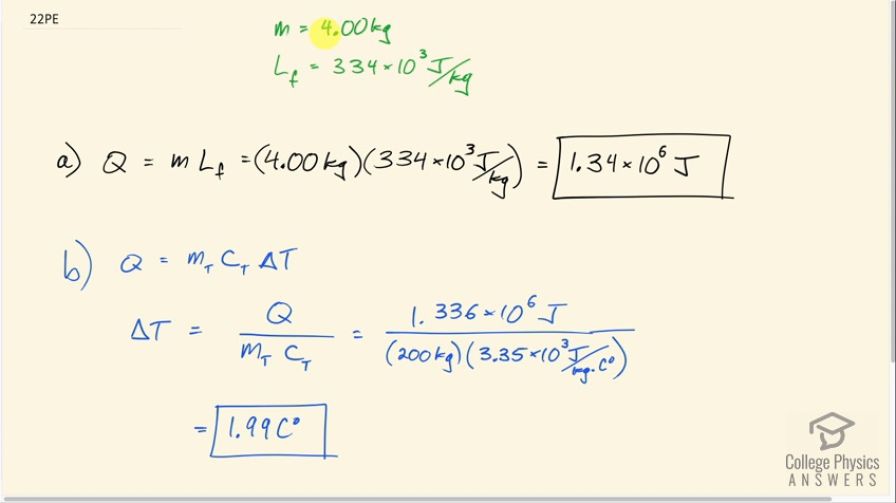
To help prevent frost damage, 4.00 kg of water is sprayed onto a fruit tree. (a) How much heat transfer occurs as the water freezes?
(b) How much would the temperature of the 200-kg tree decrease if this amount of heat transferred from the tree? Take the specific heat to be , and assume that no phase change occurs.
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 14, Problem 22 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. 4.00 kilograms of water is sprayed onto a fruit tree in order to prevent it from freezing and so the water changing phase from water to ice will release some heat to the tree and thereby prevent it from freezing. So how much heat is released from this 4.00 kilograms of water? Well that will be its mass times latent heat of fusion which is 4.00 kilograms times 334 times 10 to the 3 joules per kilogram that's 1.34 times 10 to the 6 joules. So with this amount of heat energy being given to the tree, what change in temperature did we prevent by doing this? So professional orchadists do spray their trees with water with the goal of preventing a hard frost and preventing the inside of the fruit from freezing and so in the absence of this, the temperature change that would have occurred would be the same heat lost from the tree and so it will be the mass of the tree times the specific heat of the tree divided by the change in temperature and we'll divide both sides by mass of the tree times specific heat of the tree then switch the sides around and solve for ΔT. So the change in temperature would have been 1.336 times 10 to the 6 joules divided by 200 kilograms—mass of the tree— times 3.35 times 10 to the 3 joules per kilogram per Celsius degree— specific heat of the tree— and that's 1.99 Celsius degrees.