Question
a) Find the ratio of the electrostatic to gravitational force between two electrons. (b) What is this ratio for two protons? (c) Why is the ratio different for electrons and protons?
Final Answer
- The ratio of electrostatic force to the gravitational force for the electrons and protons is different since their masses are different.
Solution video
OpenStax College Physics for AP® Courses, Chapter 18, Problem 35 (Problems & Exercises)

vote with a rating of
votes with an average rating of
.
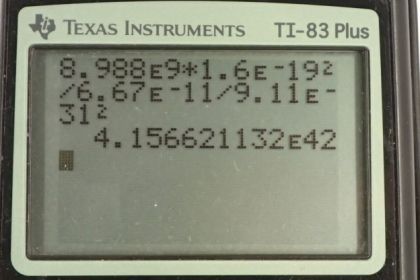
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. We're going to find the ratio of the electrostatic force to the gravitational force between two electrons and then do it again for between two protons. So the electrostatic force is Coulomb's constant times the two charges, both of which are the elementary charge and so we square them, square the elementary charge, and divide by the distance between the two charges, and the gravitational force is the gravitational constant times the mass of each electron multiplied together, divided by the distance between them squared. The ratio of these two forces is going to be the electrostatic force divided by the gravitational force. Now instead of dividing a fraction by a fraction, I'm going to multiply by the reciprocal of this fraction, so multiplying by r squared over g m e squared. This works out to k q e squared over G m e squared and the r squareds cancel. Then we substitute in numbers and we have the Coulomb's constant times the elementary charge squared, divided by the gravitational constant, times the mass of an electron squared. We get 4.16 times ten to the forty-two which is a very massive number. This means electrostatic force is way bigger than the gravitational force for electrons. Now when it comes to protons, the expression is basically going to be the same but we'll have mass of a proton down here instead of mass of an electron. So we substitute in all the same numbers as before but now the mass of electron which was 9.11 times ten to the minus thirty-one kilograms, is replaced instead with 1.67 times ten to the minus twenty-seven kilograms, mass of a proton. This works out to 1.24 times ten to the thirty-six is the ratio of the electrostatic force to the gravitational force for protons. The reason these ratios are different is because the masses are different.