Question
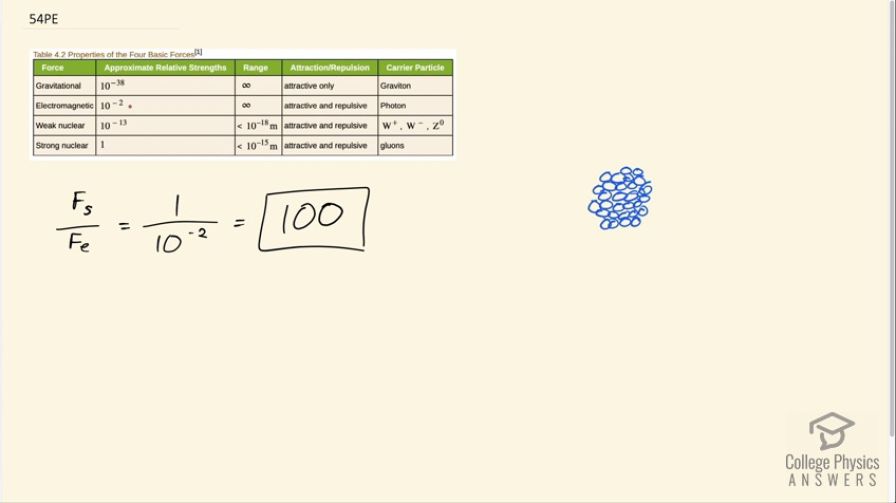
What is the ratio of the strength of the strong nuclear force to that of the electromagnetic force? Based on this ratio, you might expect that the strong force dominates the nucleus, which is true for small nuclei. Large nuclei, however, have sizes greater than the range of the strong nuclear force. At these sizes, the electromagnetic force begins to affect nuclear stability. These facts will be used to explain nuclear fusion and fission later in this text.
Final Answer
100
Solution video
OpenStax College Physics, Chapter 4, Problem 54 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Video Transcript
This is College Physics Answers with Shaun Dychko. The strong nuclear force divided by the electrostatic force is one over 10 to the minus 2, which is 100. The strong nuclear force is 100 times greater than the electrostatic force. This explains why a large nuclei are unstable. Imagine each of the circles represents a nucleon such as a proton. If you have a proton on one side of the nucleus and a proton on the other side of the nucleus, well, they're being held together because of the strong force among adjacent protons. But these two protons here are not being held together very much by the strong nuclear force and so it's so far apart. The strong nuclear force is very short range. But nevertheless, the electrostatic force still applies quite strongly because it is a longer range force. The repulsion remains but the attraction has significantly diminished because of this distance. That's why a larger nuclei become unstable and there's a certain maximum to how many nuclei it can fit into a nucleus. I should be saying nucleons instead of nuclei. Anyway, each of these is a nucleon. Okay, never mind.