Question
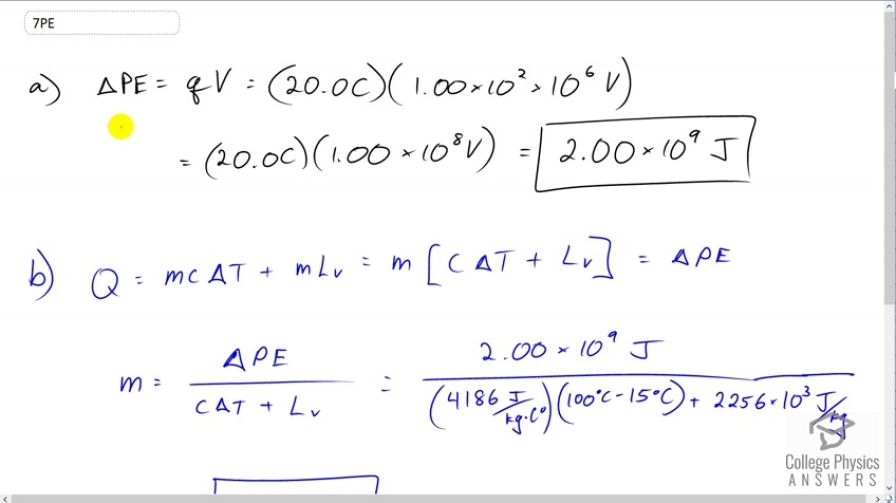
A lightning bolt strikes a tree, moving 20.0 C of charge through a potential difference of . (a) What energy was dissipated? (b) What mass of water could be raised from to the boiling point and then boiled by this energy? (c) Discuss the damage that could be caused to the tree by the expansion of the boiling steam.
Final Answer
- The tree would split apart on account of the rapidly expanding steam.
Solution video
OpenStax College Physics, Chapter 19, Problem 7 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
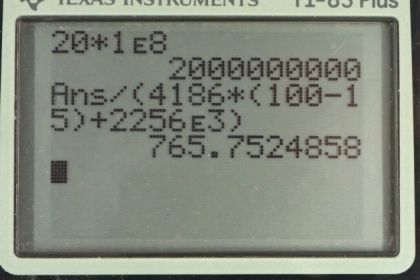
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. A lightning bolt moves 20 Coulombs of charge through potential difference of 100 Mega Volts. So the question is what energy is dissipated. So the energy through which the charges travel is going to be the charge q times the potential difference V. So that’s 20 Coulombs of charge we’re told, times one times ten to the two times ten to the six Volts, so that is one times ten to the two Mega Volts and Mega is prefix meaning times ten to the six, so we end up with times ten to the two times ten to the six. And this works out to 20 Coulombs times one times ten to the eight Volts, which is two times ten to the nine Joules. And part b asks what amount of water, what mass of water could be boiled away assuming it starts from 15 degree Celsius. So we have to look back to a previous chapter and say that the heat that the water absorbs is mass times this specific heat capacity of water times the change of temperature which is from 15 degree Celsius up to 100 degree Celsius, and then plus the energy needed to change its phase from liquid into gas. So this is mass times the latent heat of vaporization. And we can factor out the m, so we have m times c delta T plus Lv, that’s going to equal the potential energy dissipated by the lightning bolt. So we can solve for m by dividing both sides by c delta T plus Lv, and we end up with mass is potential energy divided by c delta T plus Lv. So that’s two times ten to the nine Joules, divided by 4186 Joules per kilogram Celsius degree, that’s the specific heat capacity of water, times 100 degree Celsius minus 15 degree Celsius, that’s the change in temperature, and then plus 2256 times ten to the three Joules per kilogram, latent heat of vaporization for water. This works out to 766 kilograms of water could be boiled away by that lightning bolt. And what’s going to happen, is the tree will split apart because the sap inside the tree is mostly made up of water and so much of that sap is going to turn into steam instantly basically, and that will split the tree apart.