Question
What is the potential from a proton (the average distance between the proton and electron in a hydrogen atom)?
Final Answer
Solution video
OpenStax College Physics, Chapter 19, Problem 25 (Problems & Exercises)

vote with a rating of
votes with an average rating of
.
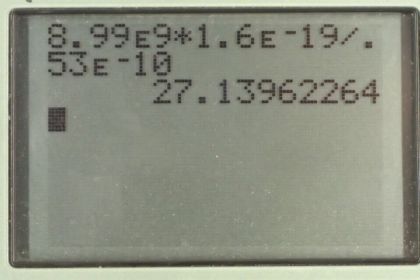
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. The potential from a point charge is K Q over R. So, Coulombs constant times the charge divided by the distance from the charge. And, this is the potential difference between the distance R from the charge and a potential of zero at infinity. And, this is going to be 8.99 times ten to the nine Newton meters squared per Coulombs squared times 1.60 times ten to the minus 19 Coulombs, because that's the charge of the proton. And, divide by 0.530 times ten to the minus ten meters, giving us a potential of 27.1 volts.