Question
An electric current through hydrogen gas produces several distinct wavelengths of visible light. What are the wavelengths of the hydrogen spectrum, if they form first-order maxima at angles of , , , when projected on a diffraction grating having 10,000 lines per centimeter? Explicitly show how you follow the steps in Problem-Solving Strategies for Wave Optics.
Final Answer
410 nm, 434 nm, 486 nm, 656 nm.
Solution video
OpenStax College Physics, Chapter 27, Problem 26 (Problems & Exercises)

vote with a rating of
votes with an average rating of
.
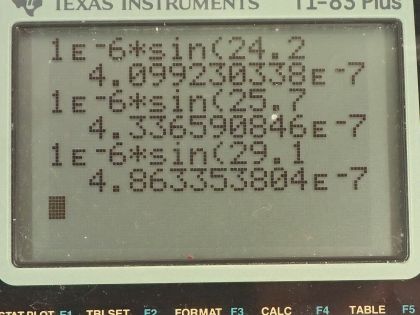
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. Electric current is passed through some pure hydrogen gas and when it does so the gas will emit some light and it will emit four different wavelengths and when that light is passed through a diffraction grating that has 10000 lines in every centimeter, the first order maximum seen on a screen behind this diffraction grating will have four different maxima— these different angles— so it will split up the light emitted into its four component colors or wavelengths. d is the amount of space between each line in a diffraction grating and so we are told there are 10000 lines in a centimeter or we can write it as 1 centimeter for every 10000 lines and then convert the centimeters into meters by multiplying by 1 meter for every 100 centimeters and then we have meters per lines and 1.00 divided by 10000 is 1.00 times 10 to the minus 6 meters per line so that's the distance between each line. We use this formula for the maxima seen after passing through a diffraction grating which is the distance between lines times sin of the angle to some maximum and then that equals the order of the maximum times the wavelength of light. So we can solve this for λ by dividing both sides by the order m and so λ is dsin Θ over m. So the first wavelength is going to be 1.00 times 10 to the minus 6 meters— that's the distance between lines and diffraction grating— times sin of that first angle 24.2 degrees divided by the order which is 1 and that is 410 nanometers. The second wavelength is the same distance— 1.00 times 10 to the minus 6 meters— times sin of the second angle 25.7 degrees and also divided by the order 1 but I just didn't bother writing it because dividing by 1 doesn't change the value and this is 434 nanometers. Wavelength 3 is the distance between lines on the diffraction grating times sin of 29.1 degrees and that is 486 nanometers and then the fourth wavelength multiplying 1.00 times 10 to the minus 6 meters times sin 41.0 degrees gives 656 nanometers.