Question
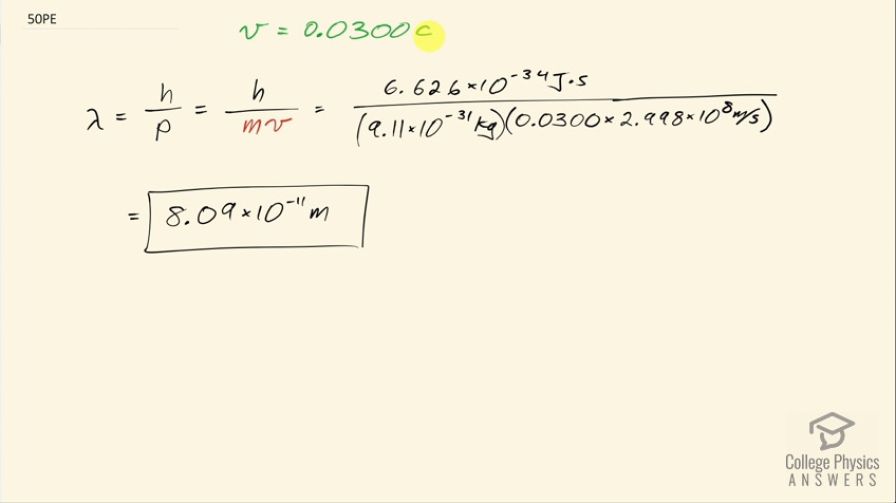
What is the wavelength of an electron moving at 3.00% of the speed of light?
Final Answer
Solution video
OpenStax College Physics, Chapter 29, Problem 50 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. What is the de Broglie wavelength for an electron traveling at the speed of 3 percent the speed of light, which is 0.0300 times c? So the wavelength is Planck's constant divided by the momentum of the electron and this speed we'll take to be slow enough that we don't need to use the relativistic expression for momentum and we'll plug in mv. So we have Planck's constant divided by the mass of an electron times its speed 0.0300 times the speed of light and that works out to 8.09 times 10 to the minus 11 meters.
