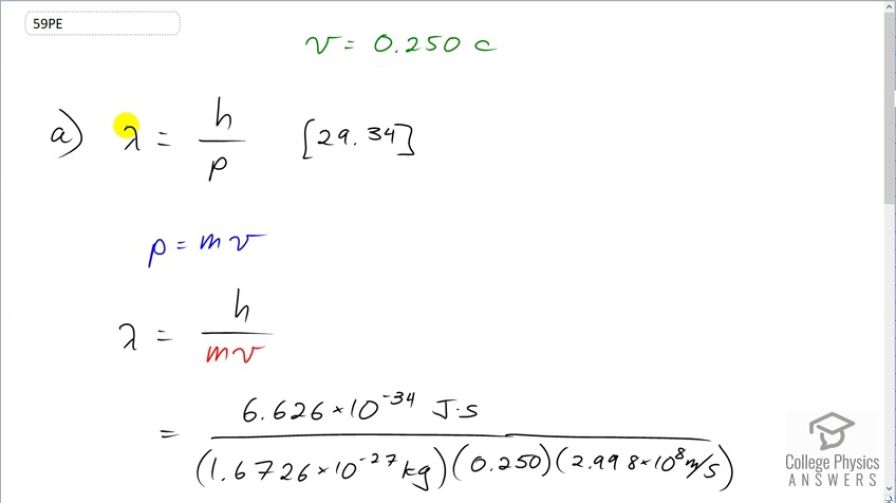
Question
The velocity of a proton emerging from a Van de Graaff accelerator is 25.0% of the speed of light. (a) What is the proton’s wavelength? (b) What is its kinetic energy, assuming it is nonrelativistic? (c) What was the equivalent voltage through which it was accelerated?
Final Answer
Solution video
OpenStax College Physics, Chapter 29, Problem 59 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. A proton emerges from a Van de Graaff accelerator with a velocity of 25 percent the speed of light and the first question is, what is its wavelength? So that is going to be Planck's constant divided by its momentum and since we are told that its velocity is non-relativistic, we'll say that the momentum is mass times velocity which we can then replace p with. So lambda then is Planck's constant over mass times velocity. So we have Planck's constant divided by the mass of a proton times 0.250 times the speed of light, which gives 5.29 femtometers, is its de Broglie wavelength. Its kinetic energy is one-half mass times velocity squared so that's one-half times mass of a proton times 0.250 times the speed of light, all squared, giving 4.7 times 10 to the minus 12 joules of kinetic energy. Then in part (c), we are asked to figure out what voltage must it have been accelerated through? Well, all of this kinetic energy was given to the proton by this potential difference and that amount of energy will be the potential difference times the charge. And so we'll divide both sides by the charge q. And so we get the voltage is the kinetic energy divided by q. So this is the kinetic energy we found in part (b), divided by the elementary charge in a proton which is 29.3 megavolts.

