Question
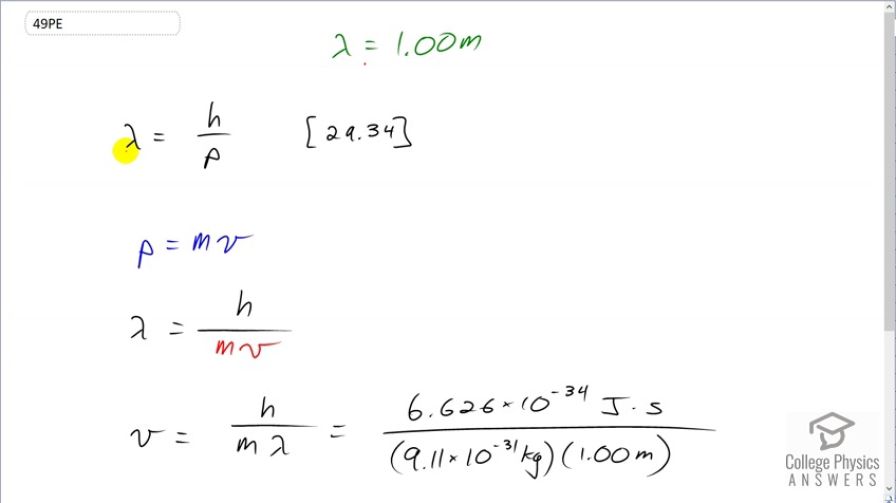
At what velocity will an electron have a wavelength of 1.00 m?
Final Answer
Solution video
OpenStax College Physics, Chapter 29, Problem 49 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. We want to know at what velocity will an electron have a wavelength of 1 meter. So we know that the de Broglie wavelength of matter is Planck's constant divided by its momentum and we know momentum is mass times velocity. And so we can substitute mv in place of p, which we have done here. And then we'll solve for v by multiplying both sides by v over λ. And so the speed then is Planck's constant divided by the mass times the wavelength. So that's Planck's constant—expressed in joule seconds— divided by the mass of an electron multiplied by its wavelength and this gives us 7.27 times 10 to the minus 4 meters per second.
