Question
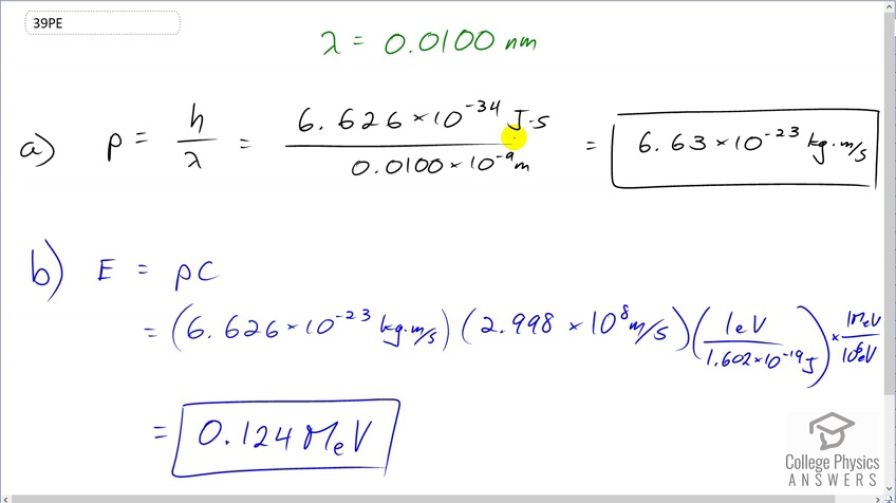
(a) What is the momentum of a 0.0100-nm-wavelength photon that could detect details of an atom? (b) What is its energy in MeV?
Final Answer
- 6.63\times 10^{-34} \textrm{ kg}\cdot \textrm{m/s}
Solution video
OpenStax College Physics, Chapter 29, Problem 39 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. The momentum of a photon is Planck's constant divided by its wavelength. So that's 6.626 times 10 to the minus 34 joule seconds divided by 0.01 times 10 to the minus 9 meters and that is 6.63 times 10 to the minus 23 kilograms meters per second. The energy of this photon is its momentum multiplied by speed of light. We have this momentum that we found in part (a) mutliplied by speed of light times 1 electron volt for every 1.602 times 10 to the minus 19 joules to turn the energy into electron volts because this will give us joules and then multiply by this many electron volts per joule. And then multiply by 1 megaelectron volt for every 10 to the 6 electron volts because this question asks us to find our answer in megaelectron volts. And that is 0.124 megaelectron volts.
