Question
What is the energy in joules and eV of a photon in a radio wave from an AM station that has a 1530-kHz broadcast frequency?
Final Answer
Solution video
OpenStax College Physics, Chapter 29, Problem 20 (Problems & Exercises)

vote with a rating of
votes with an average rating of
.
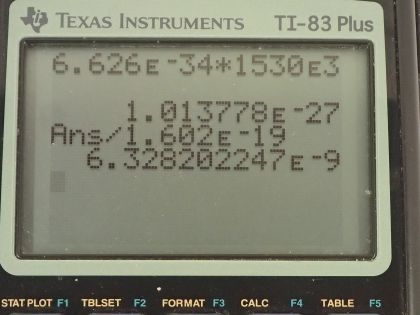
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. We are going to find the energy in units of joules and electron volts of a single photon in a radio wave from an AM station that has a frequency of 1530 kilohertz; the prefix 'kilo' means multiply by times 10 to the 3 so this is 1530 times 10 to the 3 hertz. So the energy is Planck's constant times the frequency so that's 6.626 times 10 to the minus 34 joule seconds times 1530 times 10 to the 3 hertz and this works out to 1.01 times 10 to the minus 27 joules and I took the unrounded version here multiplied it by this conversion between electron volts and joules by multiplying by 1 electron volt for every 1.602 times 10 to the minus 19 joules and the answer in electron volts is 6.33 times 10 to the minus 9 electron volts.