Question
Violet light of wavelength 400 nm ejects electrons with a maximum kinetic energy of 0.860 eV from sodium metal. What is the binding energy of electrons to sodium metal?
Final Answer
Solution video
OpenStax College Physics, Chapter 29, Problem 10 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
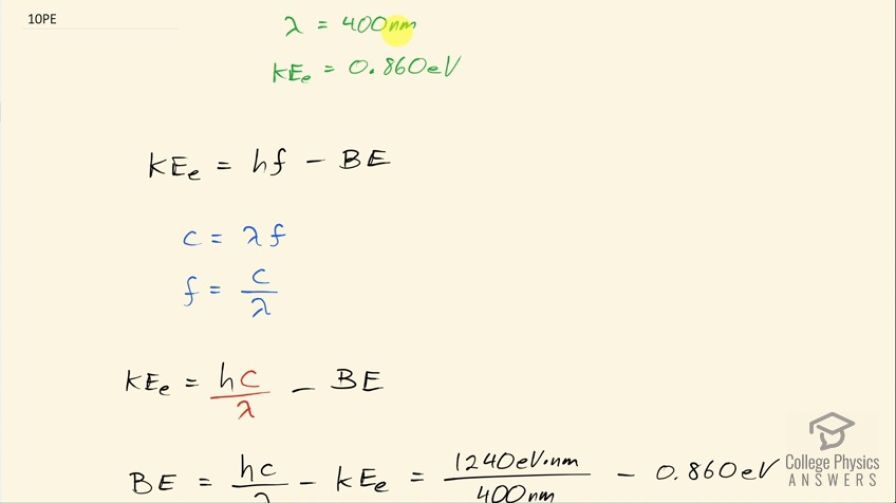
This is College Physics Answers with Shaun Dychko. Violet light with a wavelength of 400 nanometers ejects electrons from sodium metal with a maximum kinetic energy of 0.860 electron volts and the question is what is the binding energy of electrons to sodium metal? So we know that the maximum kinetic energy of the ejected electron is the total energy of the photon, which is Planck's constant times the photon's frequency minus the binding energy and first we are going to replace frequency with an expression in terms of wavelength and then we will solve for the binding energy. So we know from our wave equation that the speed of a wave equals its wavelength times its frequency and here we have the speed of the wave is its speed of light c equals λ times f so we'll divide both sides by λ and then we get f is c over λ once we switch the sides around. So we can substitute this in place of frequency and so we have this expression for the maximum kinetic energy: it's Planck's constant times speed of light divided by wavelength minus binding energy. Then we'll add binding energy to both sides and subtract the maximum kinetic energy from both sides and then we get this expression for the binding energy: it's going to be Planck's constant times speed of light divided by the wavelength minus the maximum kinetic energy; I am going to use this number— 1240 electron volt nanometers— to substitute for the product of Planck's constant times speed of light that's a convenient version of this Planck's constant to choose because then the nanometers and the units here will cancel with the nanometers that I am writing in for the wavelength— 400 nanometers— and we are left with electron volts in this term. So this many electron volts 1240 divided by 400 minus 0.860 electron volts— maximum kinetic energy— tells us what the binding energy is which is 2.24 electron volts.
