Question
What is the binding energy in eV of electrons in magnesium, if the longest-wavelength photon that can eject electrons is 337 nm?
Final Answer
Solution video
OpenStax College Physics, Chapter 29, Problem 6 (Problems & Exercises)
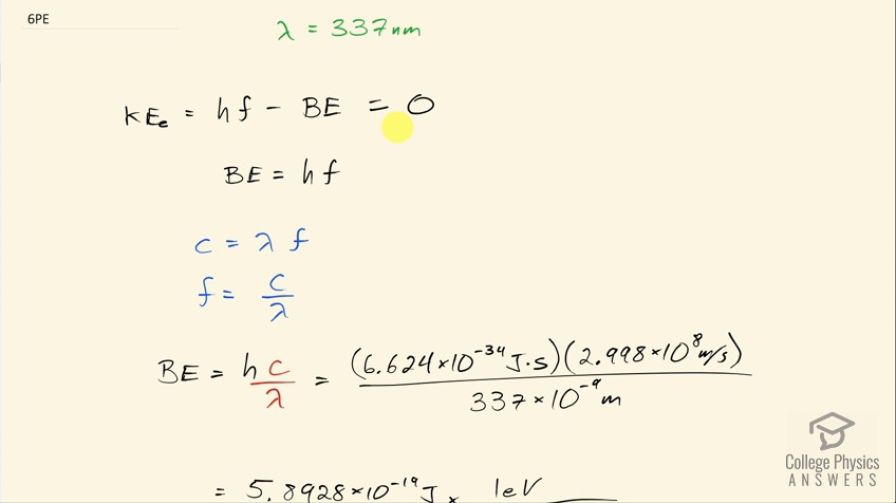
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. We are going to find the binding energy in electron volts of electrons in magnesium given that the longest wavelength photon that can eject electrons is 337 nanometers. So the longest wavelength photons have the lowest frequency because, you know, from the wave equation we know that c equals λf and so f is c over λ so f is the speed of light divided by the wavelength and so having maximizing the denominator of this fraction means we minimize the quotient or minimize frequency in other words and this term here tells us what the energy of the photon is. And so with lowering frequency with a high wavelength, we are having the lowest energy photon that can still eject an electron and so if the electron has just barely been ejected that means its kinetic energy will be at its lowest zero and so we can set this equation to zero the kinetic energy of the electron after it's been ejected is the energy of the photon minus the binding energy of the electron. So we can solve for BE by adding it to both sides and then we get to the binding energy then is Planck's constant times the frequency. So then I do this same algebra here that I did over here and figure out an expression for frequency in terms of wavelength and substitute that in for f. So the binding energy then is Planck's constant times the speed of light divided by the wavelength and in retrospect, I probably should have used 1240 electron volt nanometers in place of Planck's constant times speed of light and then divided that by 337 nanometers that would have given the same answer but anyway I did mks units here— meters, kilograms, seconds units— and we ended up with our answer in joules and then converted that into electron volts and we have 3.68 electron volts is the binding energy of an electron in magnesium.
