Question
In a nuclear fusion reaction, 2 g of hydrogen is converted into 1.985 g of helium. What is the energy released?
Final Answer
(c)
Solution video
OpenStax College Physics for AP® Courses, Chapter 32, Problem 4 (Test Prep for AP® Courses)

vote with a rating of
votes with an average rating of
.
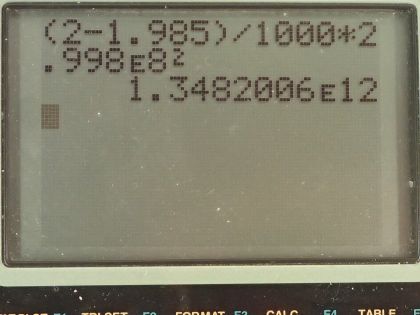
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. In a nuclear fusion reaction, 2 grams of hydrogen is converted into 1.985 grams of helium and what is the energy released? The energy released will be this difference in mass between the reactance and the products times c squared. So that's 2 grams minus 1.985 grams converted into kilograms by multiplying by 1 kilogram for every 1000 grams and then multiplied by the speed of light and that's 2.998 times 10 to the 8 meters per second squared and that's 1.35 times 10 to the 12 joules. The answer is (c).