Question
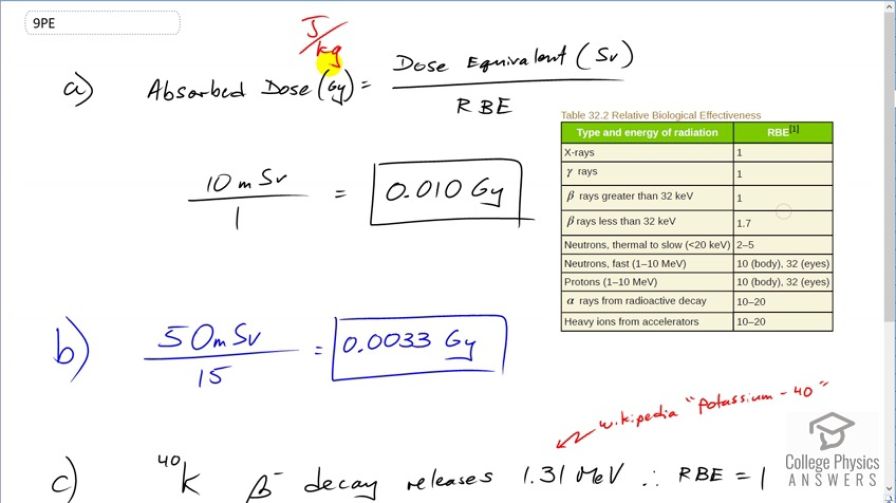
Find the radiation dose in Gy for: (a) A 10-mSv fluoroscopic x-ray series. (b) 50 mSv of skin exposure by an emitter. (c) 160 mSv of and rays from the in your body.
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 32, Problem 9 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
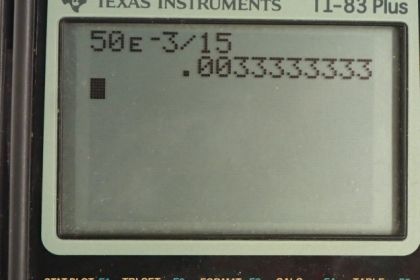
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. We are going to figure out the absorbed dose in grays which is a unit short for joules per kilogram. How much energy per kilogram is absorbed based on these different types of radiation in parts (a), (b) and (c). So in part (a), we are dealing with x-rays and we are given the dose equivalent in sieverts which is a unit that accounts for the different degrees of interaction between human tissue and these different types of radiation. according to this relative biological effectiveness. So if we take this dose equivalent in sieverts and divide it by the relative biological effectiveness of the different types of radiation then we get the dosage in grays. So in part (a), we are told we have x-rays and there's 10 millisieverts of x-rays received and we divide that by the relative biological effectiveness of x-rays which is 1 and so we have 0.010 grays. And then in part (b), we have an alpha radiation and there's 50 millisieverts absorbed of alpha radiation. An alpha radiation is a very highly interacting type of radiation because they are large particles you know consisting of two protons and two neutrons and so the relative biological effectiveness is anywhere between 10 and 20; we'll take it to be 15 in the middle of that range. So we have 50 millisieverts divided by 15 gives 0.0033 grays. And so we can see that the energy of the alpha particles is quite a bit smaller than the energy of x-rays because it doesn't take very many alpha rays to have a large impact on human tissue as a result of this high relative biological effectiveness. OK Now the comparison isn't so good because this is 10 versus 50 millisieverts but you could multiply this by 5 and you'd still see an even greater difference if you had equivalent dose equivalence in millisieverts; you would be comparing 0.05 grays to 0.0033 grays. Alright part (c) says potassium-40 undergoes beta decay and we have 160 millisieverts dose equivalent and we have to divide by the relative biological effectiveness but for beta decay, we have two different answers; 1 or 1.7 depending on the energy of the beta ray. And so in Wikipedia, I looked up the decay energy which we could have calculated but that would have taken more work and so it turns out that it's 1.31 megaelectron volts and so because that number is greater than 32 kiloelectron volts which is 0.032 megaelectron volts; clearly that number is small versus this number here for that reason, we have 1 for relative biological effectiveness. So we take 160 millisieverts divided by 1 which is 0.16 grays.