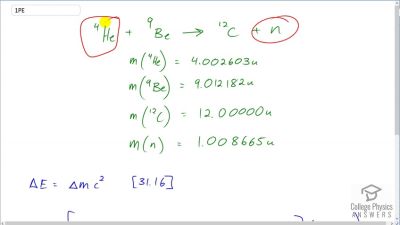
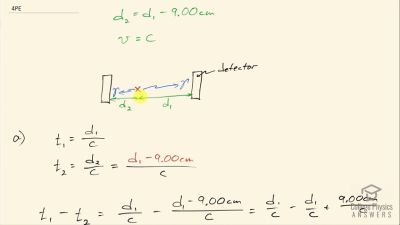
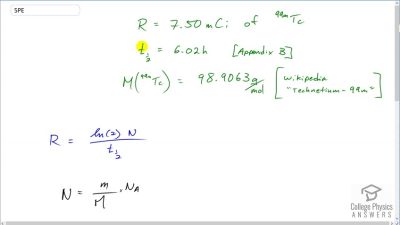
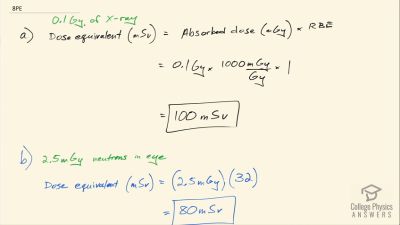
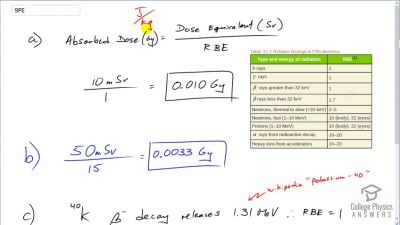
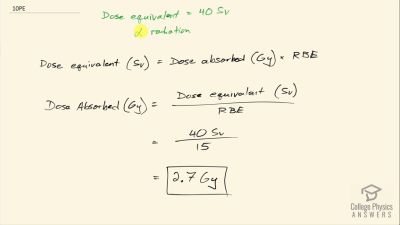
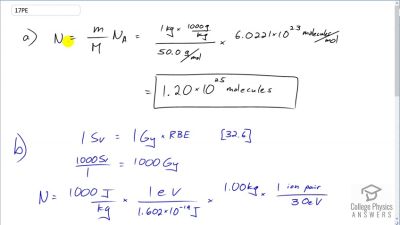
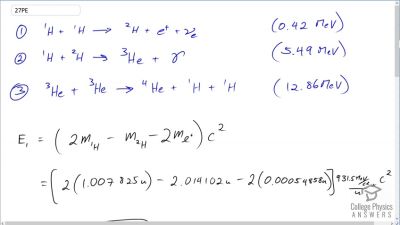
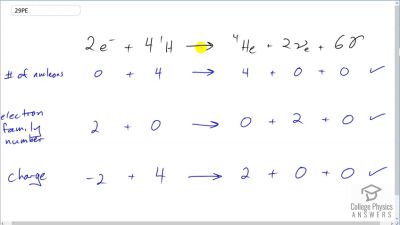
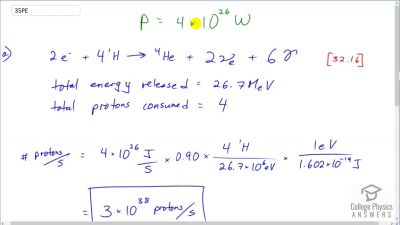
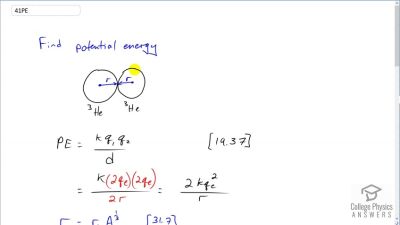
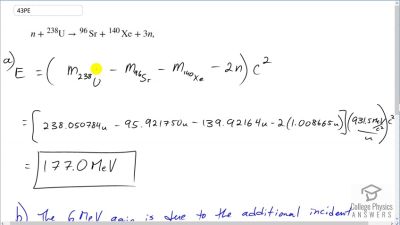
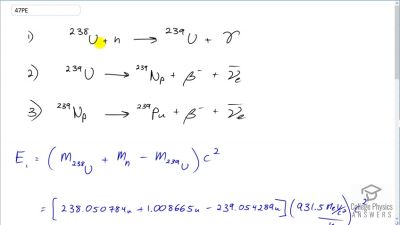
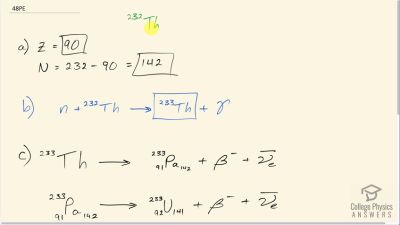
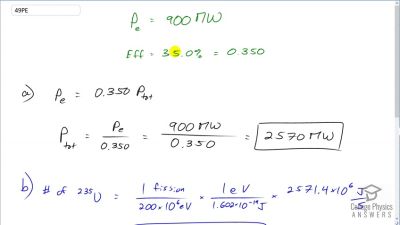
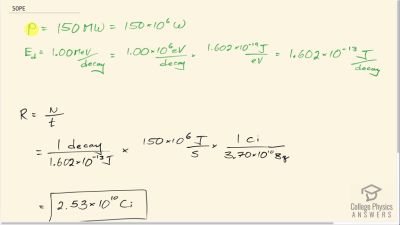
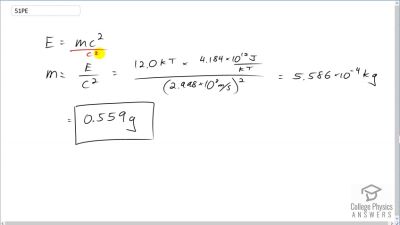
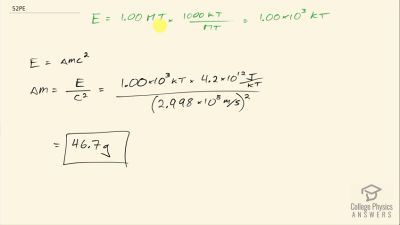
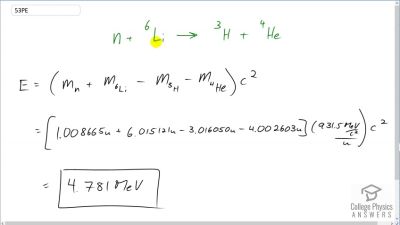
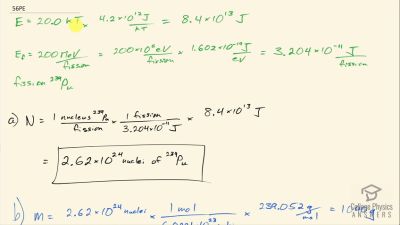
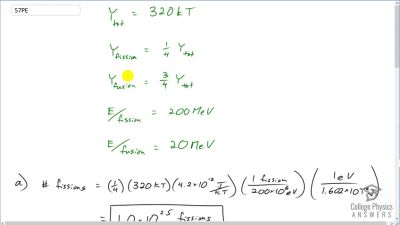
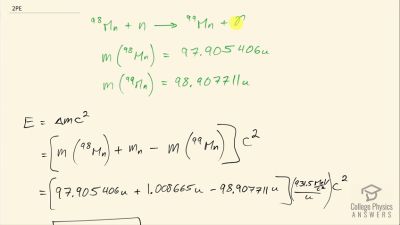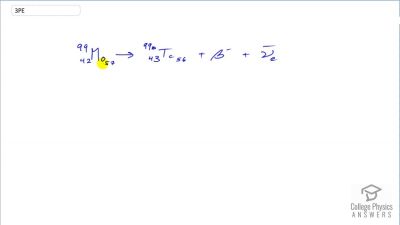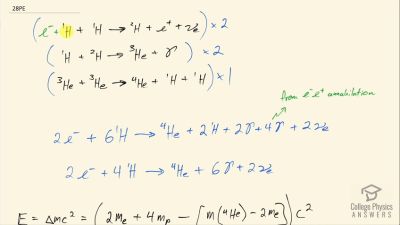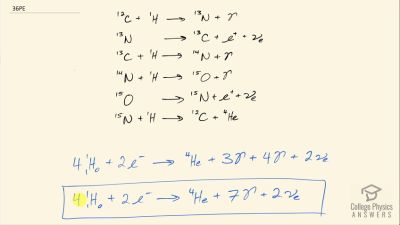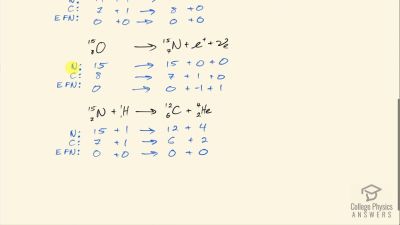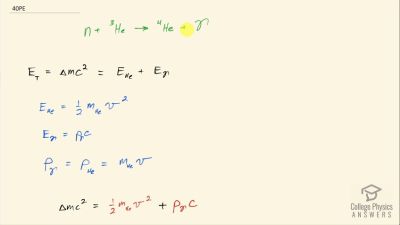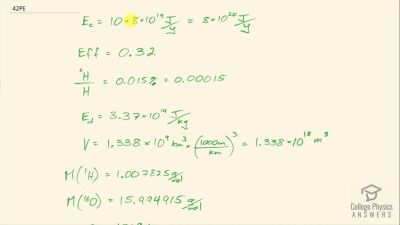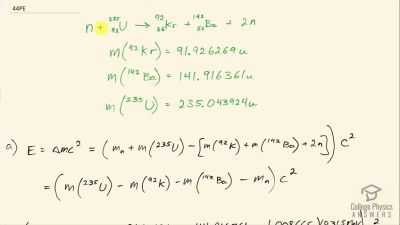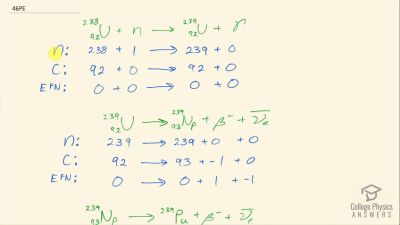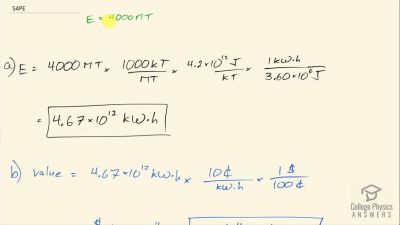Another set of reactions that result in the fusing of hydrogen into helium in the Sun and especially in hotter stars is called the carbon cycle. It is
12C+1H→13N+γ
13N→13C+e++νe
13C+1H→14N+γ
14N+1H→15O+γ
15O→15N+e++νe
15N+1H→12C+4He
Write down the overall effect of hte carbon cycle (as was done for the proton-proton cycle in
2e−+41H→4He+2νe+6γ). Note the number of protons (
1H) required and assume that the positrons (
e+) annihilate electrons to form more
γ rays.