Question
Dry air is 78.1% nitrogen. What is the partial pressure of nitrogen when the atmospheric pressure is ?
Final Answer
Solution video
OpenStax College Physics, Chapter 13, Problem 49 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
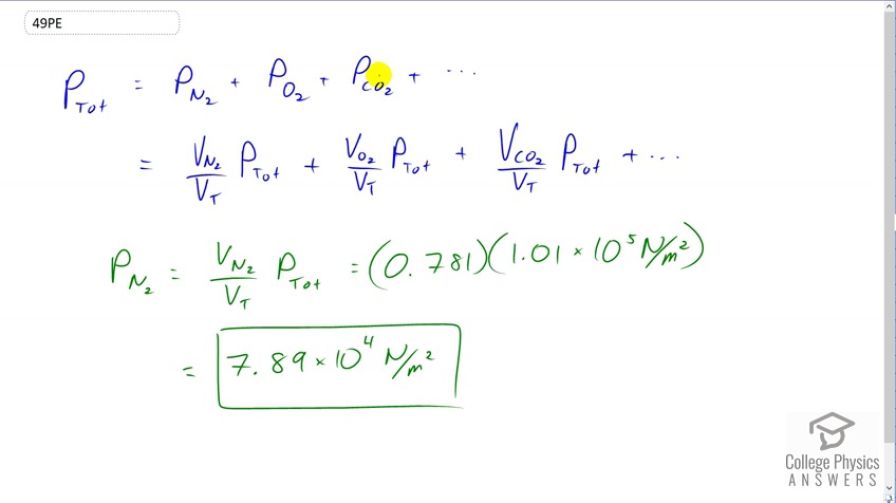
This is College Physics Answers with Shaun Dychko. The total pressure of a gas mixture is the total of the partial pressures of each of the component gases. The partial pressure of each gas is going to be the fraction of the total volume that that gas makes multiplied by the total pressure. So we're told that nitrogen takes a volume of 0.781 or 78.1 percent of the total volume of air is nitrogen. So we can multiply that percentage by the total pressure to find the partial pressure of nitrogen. So we have 0.781 times 1.01 times ten to the five newtons per square meter atmospheric pressure. This give 7.89 times ten to the four newtons per square meter is the partial pressure of the nitrogen in the air.
