Question
On a certain day, the temperature is and the relative humidity is 90.0%. How many grams of water must condense out of each cubic meter of air if the temperature falls to ? Such a drop in temperature can, thus, produce heavy dew or fog.
Final Answer
Solution video
OpenStax College Physics, Chapter 13, Problem 64 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
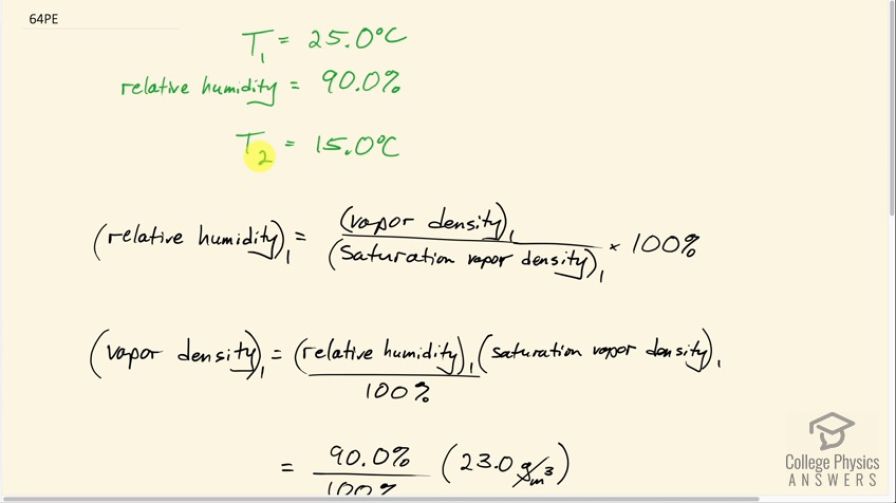
This is College Physics Answers with Shaun Dychko. At 25 degrees Celsius, there's a relative humidity of 90%. And the question says: suppose the temperature goes down to 15 degrees Celsius, how much of this moisture is going to condense out of the air? So the reason that will happen is because the saturation vapor density at 15 degrees Celsius is going to be lower than the vapor density that the air begins with. So we have relative humidity in the first place equals the first vapor density divided by the saturation vapor density at 25 degrees. And we'll use this and rearrange it to calculate what the vapor density is. And then we'll find the difference between the vapor density and the saturation vapor density at 15 degrees Celsius, saturation vapor density number two. So we'll multiply this by saturation vapor density on both sides and divide both sides by a hundred. And then we get this version here where you switch the sides around to have the unknown on the left. Vapor density one is the relative humidity divided by a hundred. So we express it as a decimal times the saturation vapor density at 25 degrees Celsius which we look up in table 13.5. So at 25 degrees Celsius, we have a saturation vapor density of 23.0 grams per cubic meter. And so 90% of that is 20.7 grams per cubic meter. So the amount that condenses is going to be the difference between this vapor density that we start with minus the maximum that's possible at the new temperature. And so at 15 degrees Celsius we see that the saturation vapor density is 12.8 grams per cubic meter. So the difference between 20.7 that we start 12.8 is 7.9 grams per cubic meter - this much moisture has to condense.
