Question
Write the complete decay equation for the given nuclide in the complete notation. Refer to the periodic table for values of Z:
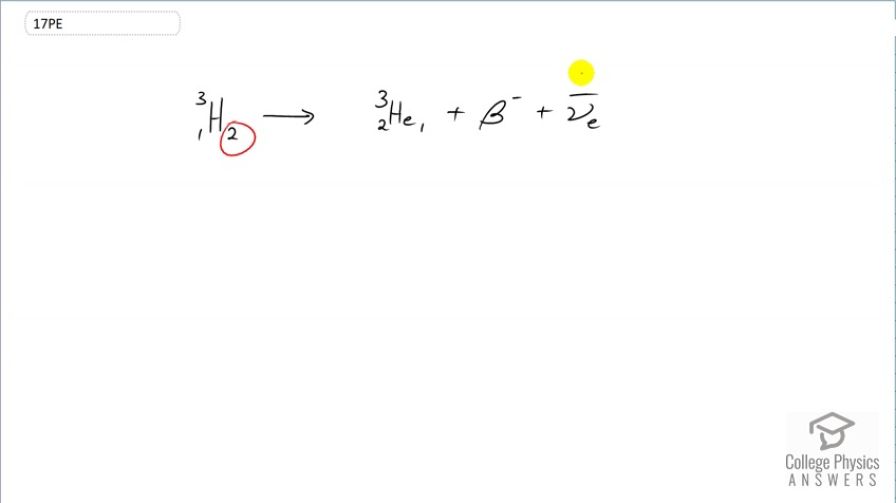
decay of (tritium), a manufactured isotope of hydrogen used in some digital watch displays, and manufactured primarily for use in hydrogen bombs.
decay of (tritium), a manufactured isotope of hydrogen used in some digital watch displays, and manufactured primarily for use in hydrogen bombs.
Final Answer
Please see the solution video.
Solution video
OpenStax College Physics, Chapter 31, Problem 17 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Video Transcript
This is College Physics Answers with Shaun Dychko. β-decay means that a neutron essentially turns into a proton with the emission of an electron So we see that the number of neutrons has decreased by 1; it goes from 2 to 1 and the number of protons has increased by 1; whereas it was 1 before, it is now 2 so it's changed from hydrogen into helium. And the number of nucleons stays the same though; total number of protons plus neutrons is still 3. In order to conserve charge in this process, an electron has to be emitted or otherwise, known as a β-particle. And in order to conserve electron family number, this β-particle has an electron family number of plus 1 so we need to have the appearance of an compensating particle that has an electron family number of negative 1 which this electron anti-neutrino has. So electron family number is conserved; charge is conserved because we have a charge of 2 in this helium nucleus and a charge of negative 1 from this β-particle which is a total of 1 just as we had in the beginning, a total of positive 1. And there we go. This is β-decay of tritium.