Question
(a) The source used in a physics laboratory is
labeled as having an activity of on the date it was
prepared. A student measures the radioactivity of this source with a Geiger counter and observes 1500 counts per minute. She notices that the source was prepared 120 days before her lab. What fraction of the decays is she observing with her apparatus? (b) Identify some of the reasons that only a fraction of the α s emitted are observed by the detector.
Final Answer
- Alpha particles are emitted radially in all directions. Only some of those alpha particles will hit the detector, and of those, only some will cause a signal.
Alpha particles are easily absorbed due to their large size and large +2 charge. Consequently some of the alpha particles produced in the interior of the sample might be absorbed by the exterior of the sample, by the air between the sample and detector, and even by the casing of the detector.
Solution video
OpenStax College Physics, Chapter 31, Problem 60 (Problems & Exercises)

vote with a rating of
votes with an average rating of
.
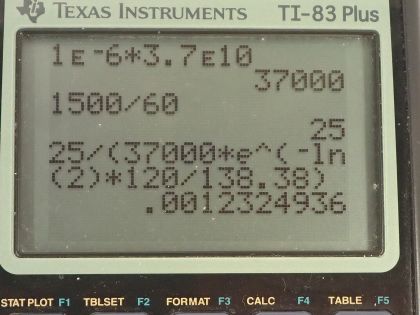
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. A sample of polonium-210 is used in a physics lab and it has an activity of 1.0 microcurie according to the label that's stamped on it on the date it was created. So now when the student measures the activity of this sample, they are measuring 1500 decays per minute which when we multiply by 1 minute for every 60 seconds is 25 becquerels whereas the original activity that it had, if converted into becquerels, by multiplying by 3.70 times 10 to the 10 becquerels per curie is 37000 becquerels. The student looks at the date on the label and it was prepared 120 days ago and we know that half-life of polonium is 138.38 days. So already we can see that there's an issue here because this amount of time past is almost equal to the half-life and so the activity should be a bit more than half of this original activity. Okay! Let's figure out the fraction of the activity that's being observed. So I am labeling this R prime, which is the activity measured by the student and this denominator here R is the activity that we are going to calculate based on knowing the original activity on the date it was prepared. So this original activity or sorry... this activity that it should have is equal to the original activity stamped on the label times e to the negative of this decay factor λ times the amount of time has passed and λ is natural logarithm of 2 divided by half-life. And so we can substitute all of this in place of R here and so we have R prime over R naught times e to the negative ln 2 times times over half-life. So that's 25 becquerels measured by the student divided by 37000 becquerels— when it was originally prepared— times e to the negative natural logarithm of 2 times 120 days that have passed divided by 138.38 days of half-life and that's 1.23 times 10 to the minus 3 so it's only a very small fraction less than about a tenth of a percent that's being detected by the student. So question (b) is asking: why is that the case? How come the student is not measuring all of the decays that are occurring? And that's because this detector has some size and it will be hit by only some of the alpha particles that are emitted by the decay— the decay occurs in all directions radially from the sample— and so this detector will only notice some of these or it will only be hit by some of these alpha particles. Now of those alpha particles that do hit the detector, only some of them will cause a signal; these are alpha particles so they don't penetrate very well, alpha particles are very big and they are strongly charged— they have a plus 2 charge— and those two things cause them to interact really easily with material. So that means an alpha particle produced in the middle of this sample might get absorbed by the polonium on the exterior of the sample so that would not be detected. And you know since like a thin sheet of paper can block alpha particles, it's possible that the casing of this detector might in fact block some of the alpha particles and prevent them from going inside the detector where it creates a signal. Okay!