Question
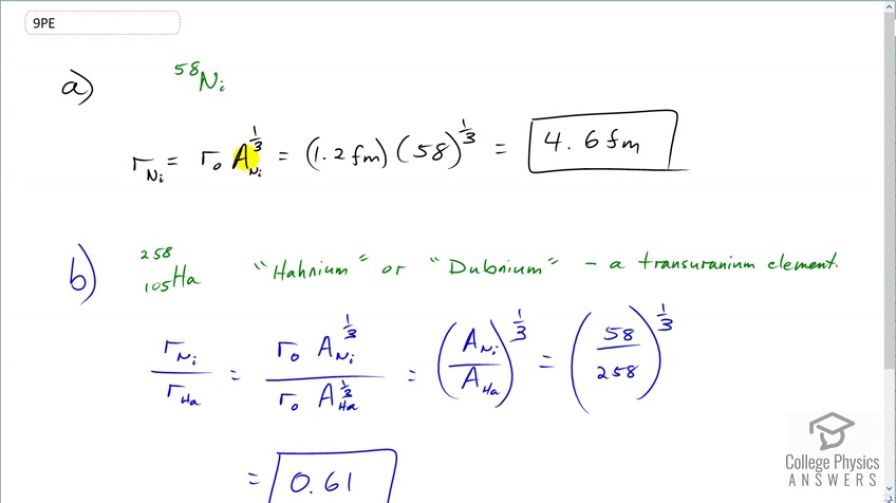
(a) Calculate the radius of , one of the most tightly bound stable nuclei. (b) What is the ratio of the radius of to that of , one of the largest nuclei ever made? Note that the radius of the largest nucleus is still much smaller than the size of an atom.
Final Answer
- $4.6\textrm{ fm}4
Solution video
OpenStax College Physics, Chapter 31, Problem 9 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. To calculate the radius of a nucleon, we use this formula—r naught times the mass number to the power of one-third. For nickel 58, there are 58 nucelons so that's the mass number, we raise that to the power of one-third multiplied by r naught which is 1.2 femtometers and this gives 4.6 femtometers. And then in part (b), we are asked to figure out the ratio of the radius of the nickel nuclide to the radius of the hahnium nuclide. So that's r naught times the mass number of nickel to the power of one-third divided by r naught times the mass number of hahnium to the power of one-third. The r naught's cancel and we can take this fraction and raise it all to the power of one-third. So that's 58 divided by 258 to the power of one-third which is 0.61.
