Question
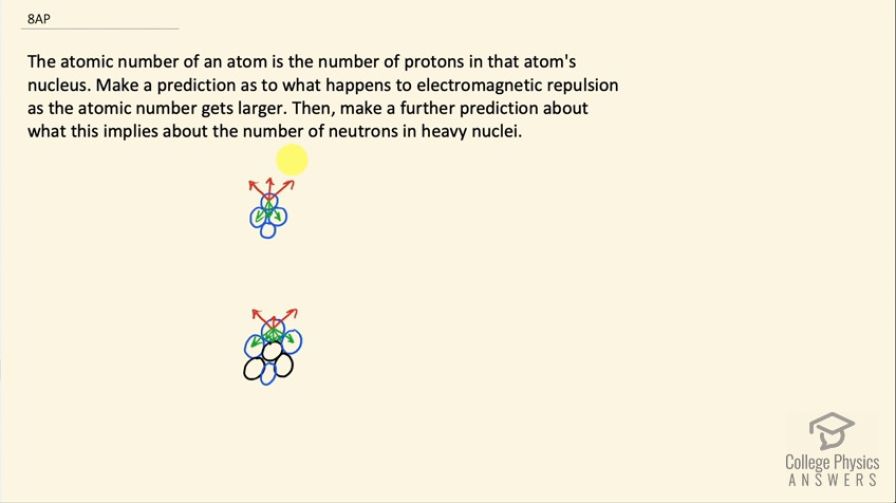
The atomic number of an atom is the number of protons in that atom's nucleus. Make a prediction as to what happens to electromagnetic repulsion as the atomic number gets larger. Then, make a further prediction about what this implies about the number of neutrons in heavy nuclei.
Final Answer
Please see the solution video.
Solution video
OpenStax College Physics for AP® Courses, Chapter 33, Problem 8 (Test Prep for AP® Courses)
vote with a rating of
votes with an average rating of
.
Video Transcript
This is College Physics Answers with Shaun Dychko. These two pictures illustrate the effect of neutrons in a nuclide. So this top picture shows the nuclide that has only four protons— each blue circle represents a proton— and there are three red arrows on this top proton to illustrate the repulsion from each of the other three protons so the arrows are directed directly away from other protons and so these two protons on this level here that are immediately touching the top proton, they each have longer arrows to represent a greater repulsive force since the proximity is so much closer whereas this bottom proton exerts a force that's repulsive directly upwards but that arrow is meant to be shorter than the other two. There are also three green arrows that represent the strong nuclear force that is attractive and it is longest and strongest towards the protons that are actually touching the top proton and then it's a little bit shorter because of the distance to the proton at the bottom here. Now when it comes to the strong nuclear force, distance makes a big difference. So the strong nuclear force applies only on the scale of a nuclide and so you know, a proton on the opposite side of a nuclide will have almost no strong attractive force to other protons on the other side of the nuclide but the electrostatic repulsion however will still apply because its range is infinite. Okay! So now we have this bottom picture here where there are some black circles that represent neutrons and these neutrons do not repel the protons because they are neutral they have only the strong nuclear force of attraction and so this top proton now, it has the same three red arrows illustrating repulsion from the other three protons— this nuclide has the same number of protons as the first picture— but now there are additional green arrows representing additional strong nuclear force to these introduced neutrons. And actually this arrow that's straight down should be a little bit longer to illustrate how this neutron here is actually touching the top proton and so it's going to exert a very strong, strong nuclear force. So we have three long green arrows and two rather short green arrows to represent the attraction to these more distant neutrons and then it still has the same three electrostatic repulsion arrows and so we can see that there's a greater cohesion here, greater stability and so as the atomic number gets larger or in other words as more protons are added, there needs to be more neutrons to act as glue to introduce more strong nuclear force to compensate for the electrostatic repulsion that's going to be increasing as more protons are added.