Question
What is the smallest-wavelength line in the Balmer series? Is it in the visible part of the spectrum?
Final Answer
This is not visible. It is ultraviolet.
Solution video
OpenStax College Physics, Chapter 30, Problem 15 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
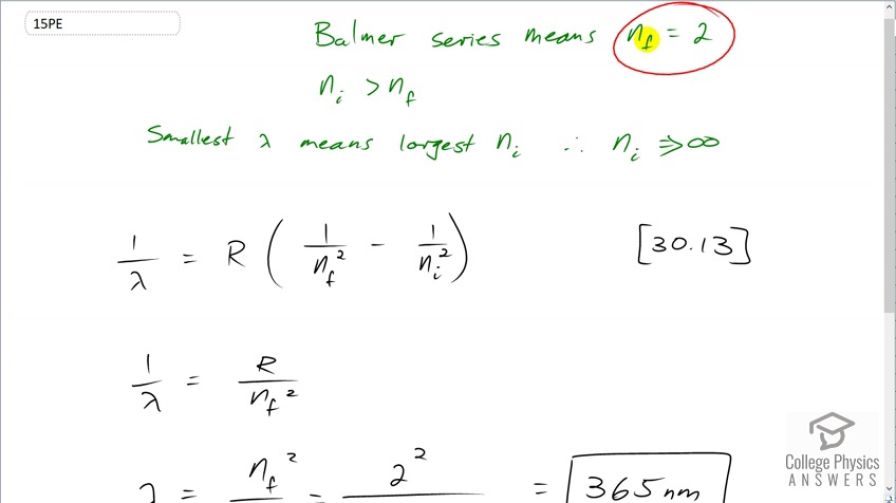
This is College Physics Answers with Shaun Dychko. We want to find the shortest wavelength emission in the spectrum of hydrogen given a final energy level of 2 which is what the Balmer series means and this n f is in this formula here which is [30.13] which says the reciprocal of the wavelength that is emitted is Rydberg's constant times 1 over the final energy level squared minus 1 over the inial energy level squared. Now we want to know what is the shortest wavelength which is the highest energy wavelength which means we want the transition to be as big as possible. And so n f is specified as 2 and so we have to choose a value of n i such that we get the shortest wavelength and we want n i then to be as big as possible which will essentially make it infinity. So this will be the largest transition an electron comes from close to infinity to this energy level of 2 and that transition will be the highest energy or in other words, the shortest wavelength transition. So with n i being essentially infinity that means 1 divided by that will be 0 and so that means this reduces to just R over n f squared. And then solving for λ then means we flip both sides or raise both sides to exponent negative 1 and λ is n f squared over Rydberg's constant which is 2 divided by 1.097 times 10 to the 7 reciprocal meters which gives 365 nanometers. This is not visible; 380 nanometers is the shortest wavelength we can see which is violet and this is slightly shorter than that so this is ultraviolet.
