Question
(a) What is the shortest-wavelength x-ray radiation that can be generated in an x-ray tube with an applied voltage of 50.0 kV? (b) Calculate the photon energy in eV. (c) Explain the relationship of the photon energy to the applied voltage.
Final Answer
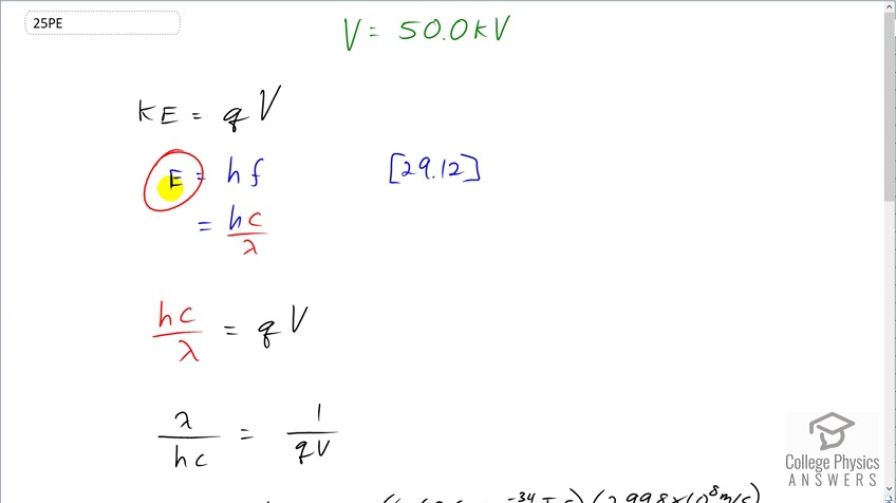
- The maximum photon energy is the kinetic energy of the electron, which is . This is the electron charge times Voltage, which is "electron-volts". Therefore, the maximum photon energy is the x-ray tube voltage written in "eV".
Solution video
OpenStax College Physics, Chapter 30, Problem 25 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. A 50 kilovolt x-ray tube accelerates electrons and the maximum energy of the photon emitted by this x-ray tube will be equal to the kinetic energy of the electrons. And that's gonna be the electron charge multiplied by the voltage through which it is accelerated. And so we can equate the energy which we can write as Planck's constant times speed of light divided by lambda and you can equate that to qV. And let's solve this for lambda by taking the reciprocal of both sides so this λ over hc equals 1 over qV and then multiply both sides by hc. So we have the shortest possible wavelength is hc over qV. So that's 6.626 times 10 to the minus 34 joule seconds—Planck's constant— times speed of light divided by elementary charge times 50 times 10 to the 3 volts giving 2.48 times 10 to the minus 11 meters is the shortest possible wavelength. And then in part (b), we calculate the energy of this wavelength and that's hc divided by λ and hc we are going to take to be 1240 electron volt nanometers and so we'll divide by this wavelength also expressed in units of nanometers so that these nanometers cancel and we'll get an answer in electron volts. So this written in nanometers is 0.02480 nanometers and this works out to 50.0 kiloelectron volts. And in part (c), we talk about how this corresponds to this this is the voltage of the x-ray tube— 50 kilovolts— and this is the maximum energy photon emitted and the maximum photon energy is the kinetic energy of the electron which is q times V and this is the elementary charge of an electron multiplied by the voltage so that makes electron volts and therefore, we see that the maximum photon energy is the x-ray tube voltage written in units of electron volts. So where we started with 50 kilovolts, we can write that as 50 kiloelectron volts for calculating the maximum photon energy.
