Question
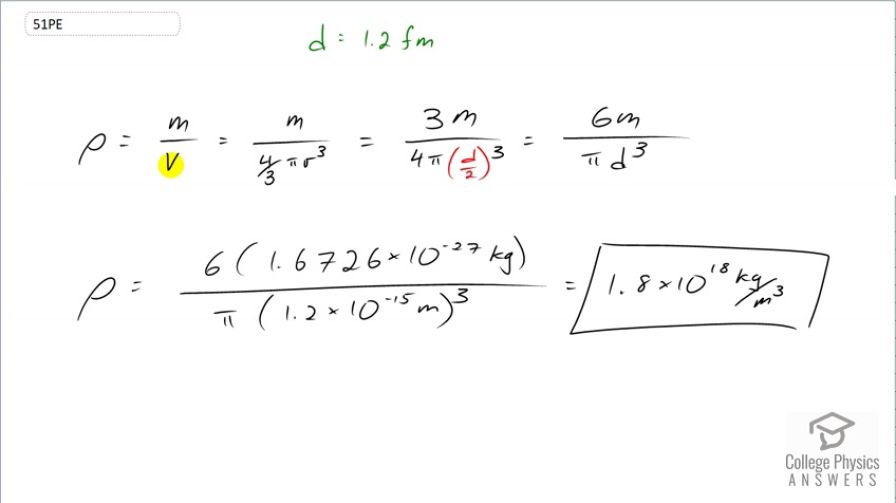
Estimate the density of a nucleus by calculating the density of a proton, taking it to be a sphere 1.2 fm in diameter. Compare your result with the value estimated in this chapter.
Final Answer
Solution video
OpenStax College Physics, Chapter 30, Problem 51 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. We are going to calculate the density of a proton assuming it has a diameter of 1.2 femtometers. So density is mass divided by volume and we'll take the proton to be a sphere in which case the volume is four-thirds π times radius cubed and then multiply top and bottom by 3; these 3's cancel leaving us a 3 on top. So we have 3m over 4π times radius cubed but we'll write radius as diameter divided by 2 since we are given diameter and we'll cube that and in which case, we have a denominator of 8 because 2 cubed is 8 and 4 with that 8 in the denominator there makes a denominator of 2 underneath d cubed and multiply top and bottom by 2, we end up with 6m over π d cubed. So the density then is 6 times the mass of a proton divided by π times the diameter of a proton cubed which is 1.8 times 10 to the 18 kilograms per cubic meter.
