Question
A color television tube also generates some x rays when its electron beam strikes the screen. What is the shortest wavelength of these x rays, if a 30.0-kV potential is used to accelerate the electrons? (Note that TVs have shielding to prevent these x rays from exposing viewers.)
Final Answer
Solution video
OpenStax College Physics, Chapter 30, Problem 26 (Problems & Exercises)
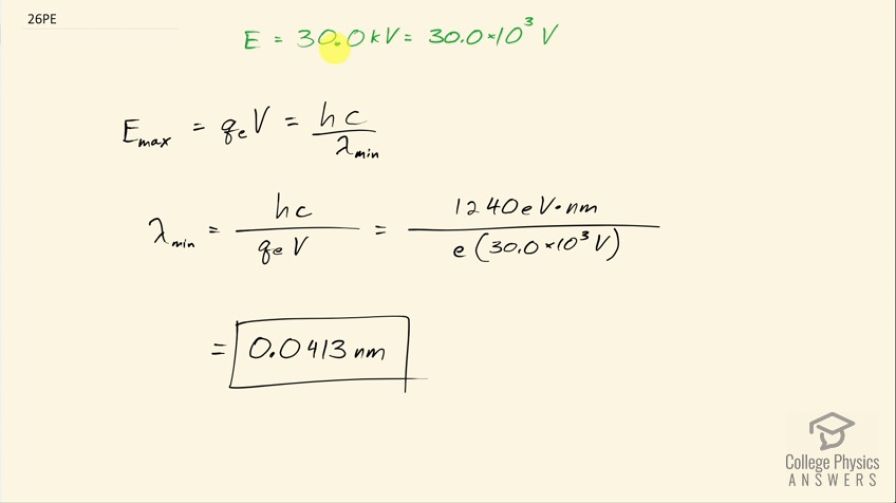
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. Electrons are accelerated in a cathode ray tube of an old-fashioned television and the accelerating potential is 30.0 kilovolts and the question is what is the shortest wavelength x-ray that could possibly be produced by this electron? So when the electron decelerates, the maximum possible energy photon that it could produce will be equal to the kinetic energy that it has and the kinetic energy that the electron has is equal to the potential energy that it lost and the potential energy it lost is the charge on the electron multiplied by the potential difference. And so that at its most will be the energy of the photon which is Planck's constant times speed of light divided by the wavelength of the photon. Now the maximum energy will occur when the wavelength is at its minimum so this is what we have to solve to find the minimum possible wavelength. So I'll multiply both sides by λ min divided by charge on the electron multiplied by V and we do the same on this side here. So the minimum wavelength then is hc over the charge on the electron times the voltage; I am taking bit of shortcuts with units here and I have written 1240 electron volt nanometers in place of Planck's constant times c and dividing by the charge on the electron, which is this elementary charge e multiplied by 30.0 times 10 to the 3 volts and the product of these will give units of electron volts because that's what an electron volt is— it's the charge of an electron multiplied by a volt. So these electron volts cancel leaving us with units of nanometers and this works out to 0.0413 nanometers will be the shortest wavelength x-ray produced by the deceleration of this electron accelerated through 30.0 kilovolts.
