Question
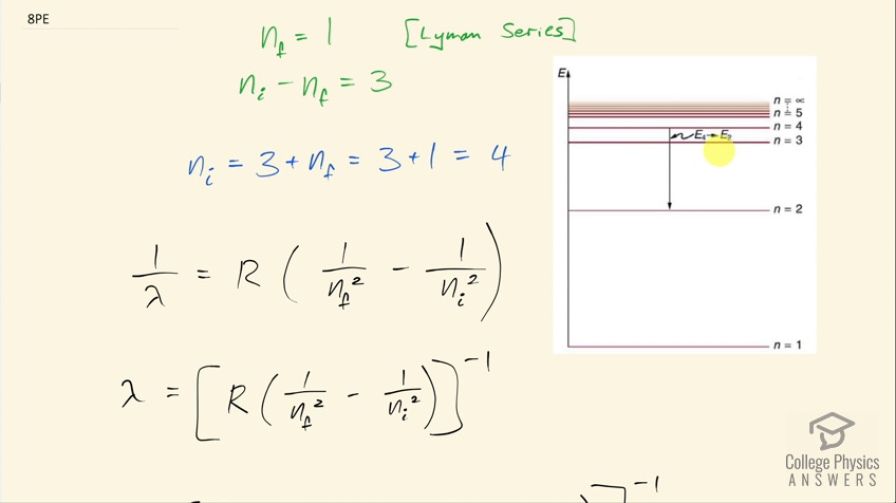
Find the wavelength of the third line in the Lyman series, and identify the type of EM radiation.
Final Answer
This is ultraviolet.
Solution video
OpenStax College Physics, Chapter 30, Problem 8 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
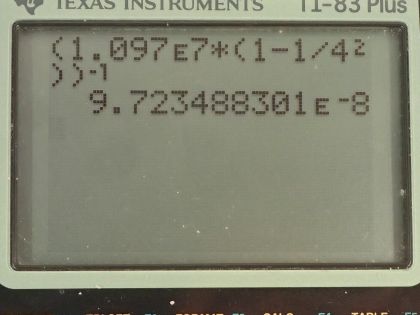
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. We are going to find the wavelength of the third line in the Lyman Series in the emission spectrum from hydrogen. So a Lyman Series means that the final state of the electron is this first orbital n equals 1 and the third line will mean that n initial is 4 so it will start at this energy state and end at n 1 and it will emit some photon and we are going to figure out what the wavelength of that photon is. So the initial minus the final orbital is going to be 3 that's what it means to say the 3rd line because if it started at n equals 2 and went to n equals 1 that would be the first line and then this is the second line and then here is the third line. Okay! So n initial is 3 plus the final 1 for a total of 4. Then we have this formula here which tells us the reciprocal of the wavelength emitted is this Rydberg's constant times 1 over the final orbital squared minus 1 over the initial orbital squared and we'll take the reciprocal of both sides in order to get λ. So the wavelength emitted then is 1.097 times 10 to the 7 reciprocal meters times 1 over 1 squared minus 1 over 4 squared and then all that to the negative 1 gives 97.2 nanometers will be the wavelength of the photon emitted. Ultraviolet light has wavelengths from 10 nanometers upto 380 nanometers and so 97.2 nanometers falls into this range for ultraviolet light.