Please see the solution video.
Solution video
OpenStax College Physics, Chapter 30, Problem 10 (Problems & Exercises)
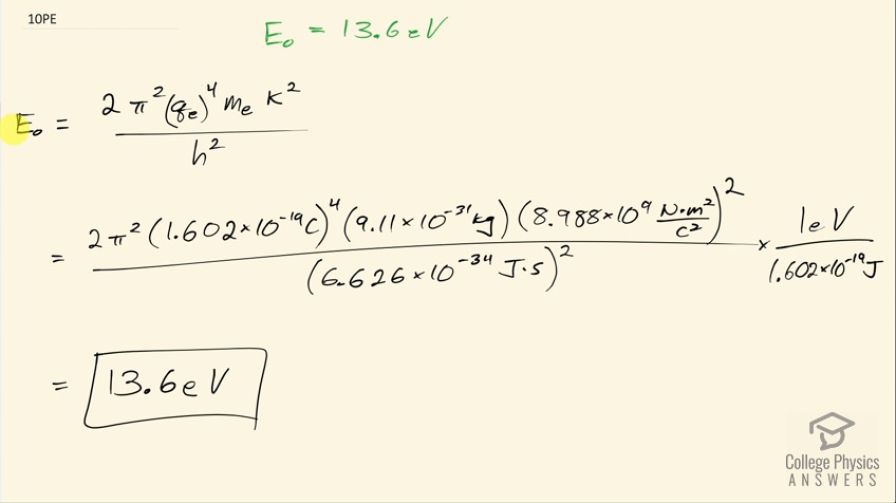
Calculator Screenshots
Comments
would this only be the ground state energy for hydrogen? does every element have its own ground state energy?
Hi Ryan, thanks for the question. is the ground state only for hydrogen, whereas other "hydrogen-like" atoms have ground states in terms of of , where I substituted for the ground state in equation [30.26] , and is the number of protons in the atom. The electron is more tightly bound to nuclei with more protons. Equation [30.26] is pretty limited, however, since it's only for "hydrogen-like" atoms, which means there is only one electron. A better term for them would be "hydrogen-like ions", rather than atoms, since, with the exception of hydrogen, they would all be charged. When additional electrons surround the nucleus (it's no longer a hydrogen-like ion in other words), things get more complicated and there doesn't exist a formula for calculating the ground state energy of the inner-most election in that circumstance. You might find the Wikipedia discussion of ionization energy interesting. The "ground state energy of the hydrogen atom" is the same as the "ionization energy of hydrogen".
All the best,
Shaun


