Question
(a) What is the maximum energy in eV of photons produced in a CRT using a 25.0-kV accelerating potential, such as a color TV? (b) What is their frequency?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 29, Problem 28 (Problems & Exercises)
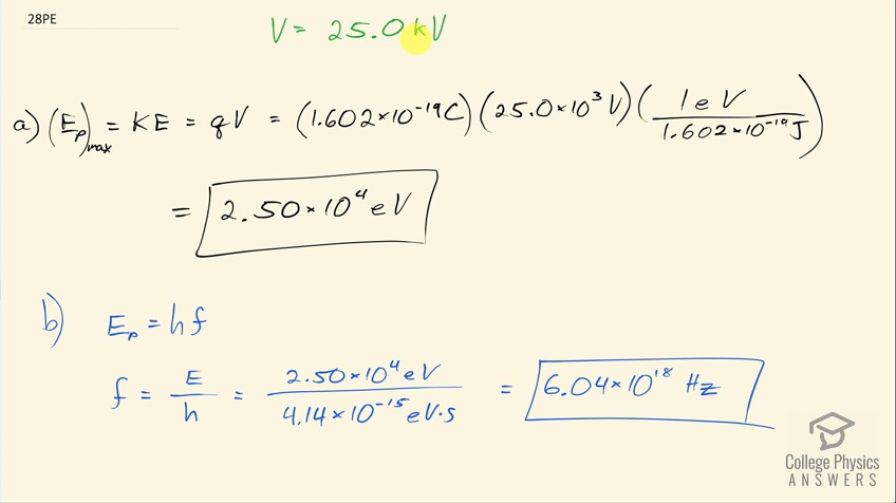
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. A cathode ray tube has an accelerating potential of 25.0 kilovolts and the question is what is the maximum possible energy photon that can be produced in this CRT? So the maximum possible photon energy will be the kinetic energy that the electron has right before it collides with the anode at the end of the cathode ray tube some of the photons will have energies less than this kinetic energy but at a maximum, it can be at the most equal to the kinetic energy. The kinetic energy the photon will acquire is equal to the charge of the photon multiplied by the potential through which it's accelerated so this is the drop in potential energy of the electron equals the gain in kinetic energy that it has. So that's the charge of the electron in coulombs multiplied by the potential difference— 25.0 times 10 to the 3 volts— and then convert it into electron volts by multiplying by 1 electron volt for every 1.602 times 10 to the minus 19 joules, this works out to these numbers canceling and being left with this number in units of electron volts so that's 2.50 times 10 to the 4 electron volts. Part (b) asks us to find the frequency of this maximum energy photon and we'll divide both sides of this formula by Planck's constant and then we get the frequency then is the energy of the photon divided by Planck's constant so that's 2.50 times 10 to the 4 electron volts divided by Planck's constant written in units of electron volt seconds that is 4.14 times 10 to the minus 15 electron volt seconds, the electron volts cancel leaving us with reciprocal seconds as our units which is abbreviated as hertz, which is the unit for frequency, and that is 6.04 times 10 to the 18 hertz.
