Question
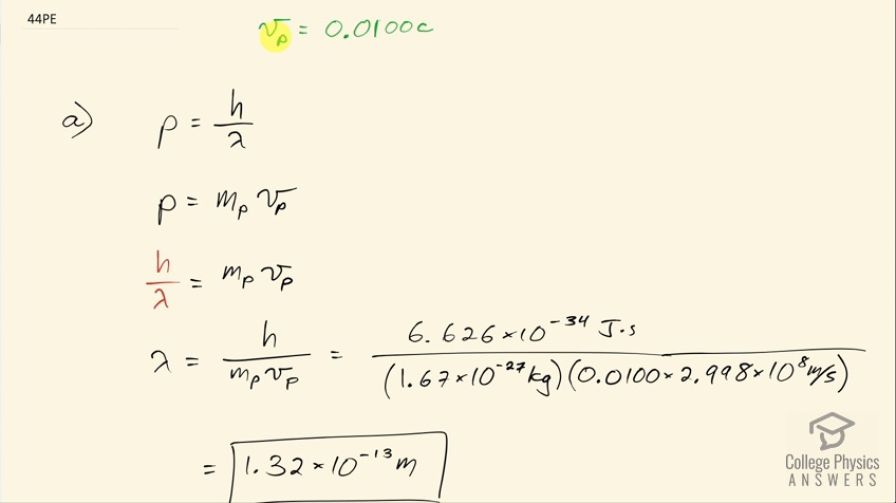
(a) Calculate the wavelength of a photon that has the same momentum as a proton moving at 1.00% of the speed of light. (b) What is the energy of the photon in MeV? (c) What is the kinetic energy of the proton in MeV?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 29, Problem 44 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. Let's calculate the wavelength of a photon that would have the same momentum as a proton that's moving at 1 percent the speed of light. So this is the velocity of the proton here; the subscript p in this case stands for 'proton.' So the momentum of a photon is Planck's constant divided by its wavelength and that same momentum in terms of the proton is going to be the mass of the proton times the velocity of the proton. So we have h over λ then equals mass of the proton times its velocity and we'll solve this for λ by multiplying both sides by λ over mass of the proton times velocity of the proton and then switch the sides around and we get λ then is Planck's constant divided by mass of the proton times velocity of the proton. So that's 6.626 times 10 to the minus 34 joule seconds divided by the mass of the proton times 0.0100 times the speed of light and that is 1.32 times 10 to the minus 13 meters. What is the energy of the photon in megaelectron volts is the next question? So the photons energy is Planck's constant times speed of light divided by wavelength and I am taking Planck's constant times speed of light to be 1240 electron volt nanometers— this is a convenient way of writing h times c and it requires the wavelength to be written in units of nanometers as well— and then we'll get an answer in electron volts and then we'll convert that into megaelectron volts by multiplying by 1 megaelectron volt for every 10 to the 6 electron volts. So we are dividing this numerator by 1.32 times 10 to the minus 13 meters converted into nanometers by multiplying by 10 to the 9 nanometers for every meter, this works out to 9.37 megaelectron volts. And what is the kinetic energy of the proton in megaelectron volts? So the kinetic energy the proton would have had is one-half times the mass of the proton times its speed squared. So that's one-half times proton mass times 1 percent of the speed of light squared and then we convert this into megaelectron volts by doing first the conversion into electron volts and then multiply by 1 megaelectron volt for every 10 to the 6 electron volts and this is 4.68 times 10 to the minus 2 megaelectron volts.

