Question
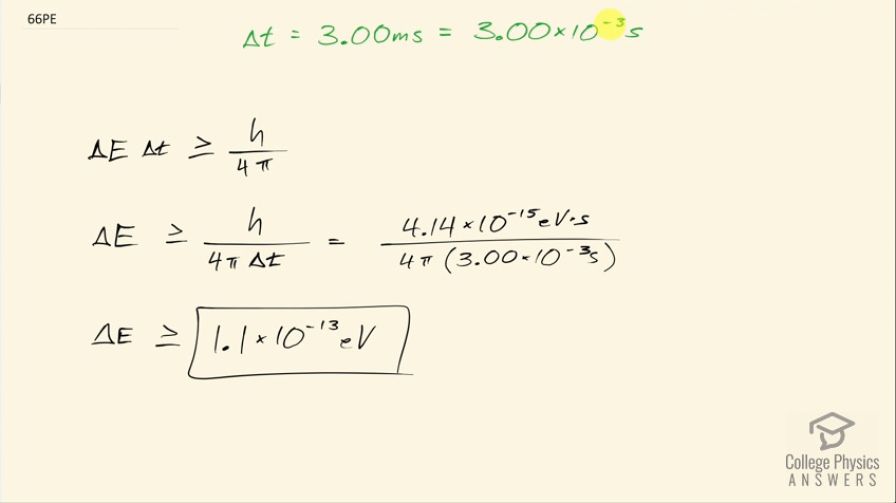
A relatively long-lived excited state of an atom has a lifetime of 3.00 ms. What is the minimum uncertainty in its energy?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 29, Problem 66 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. The uncertainty in the duration of this excited state of an atom is 3.00 milliseconds, which is 3.00 times 10 to the minus 3 seconds and the question here is what is the uncertainty then in the energy of that state? So the uncertainty in energy times the uncertainty in time is greater than or equal to Planck's constant over 4π and we can solve for ΔE by dividing both sides by Δt. So the uncertainty in energy then is Planck's constant over 4π times Δt and that is 4.14 times 10 to the minus 15 electron volt seconds where I have taken these units for Planck's constant just as a convenience because the seconds here will cancel with the seconds in the denominator and we'll be left with our units in electron volts which is what we want in the end I could have used 6.626 times 10 to the minus 34 joule seconds and then converted it afterwards into electron volts but this just saves the hassle of doing that by choosing this as our value for the constant. So we take that divide by 4π times the uncertainty in time 3.00 times 10 to the minus 3 seconds and so the uncertainty in energy is greater than or equal to 1.1 times 10 to the minus 13 electron volts.
