Question
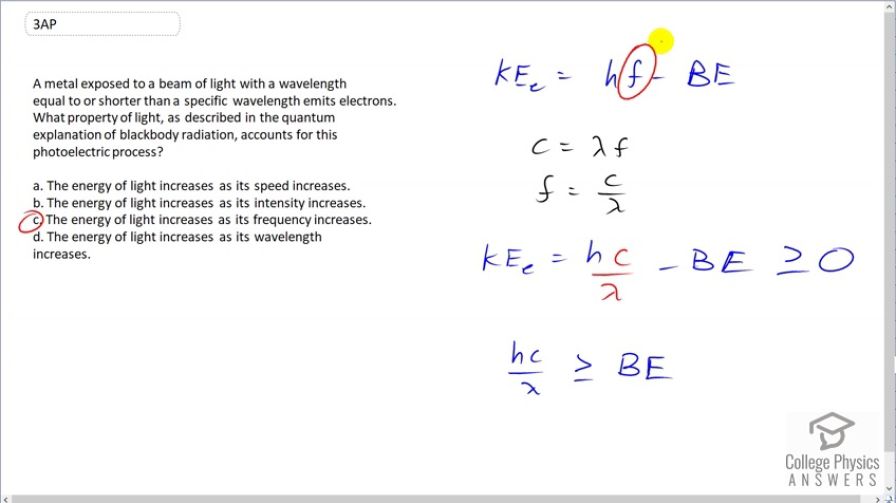
A metal exposed to a beam of light with a wavelength equal to or shorter than a specific wavelength emits electrons. What property of light, as described in the quantum explanation of blackbody radiation, accounts for this photoelectric process?
- The energy of light increases as its speed increases.
- The energy of light increases as its intensity increases.
- The energy of light increases as its frequency increases.
- The energy of light increases as its wavelength increases.
Final Answer
(c)
Solution video
OpenStax College Physics for AP® Courses, Chapter 29, Problem 3 (Test Prep for AP® Courses)
vote with a rating of
votes with an average rating of
.
Video Transcript
This is College Physics Answers with Shaun Dychko. A metal will emit electrons when it's hit by photons that have a certain frequency that exceeds the well, when multiplied by the Planck's constant, that product exceeds the binding energy, in which case, this kinetic energy will be greater than or equal to 0. And when this frequency is less than the binding energy then the electron will not be emitted at all. And we can substitute for wavelength here to see how wavelength affects things. And we have the wave equation that says the wavelength times frequency is the speed of light and solve this for f by dividing both sides by λ and then substitute c over λ in place of f. And here we see that there's a certain maximum wavelength that will still cause photoelectrons to be ejected from the metal atoms and that maximum occurs when hc over λ max equals the binding energy. And for wavelengths less than that, we are therefore increasing frequency. And because it says shorter or equal to some specific wavelength will cause electrons to be emitted and as lambda gets smaller and smaller, we can see that frequency is increasing. And so we wanna look for an answer here that talks about how frequency is increasing. and as frequency increases, the energy of the photon increases because that's what hf is and it's exceeding the electron binding energy of the material and therefore causing electrons to be emitted. The answer is (c).