Question
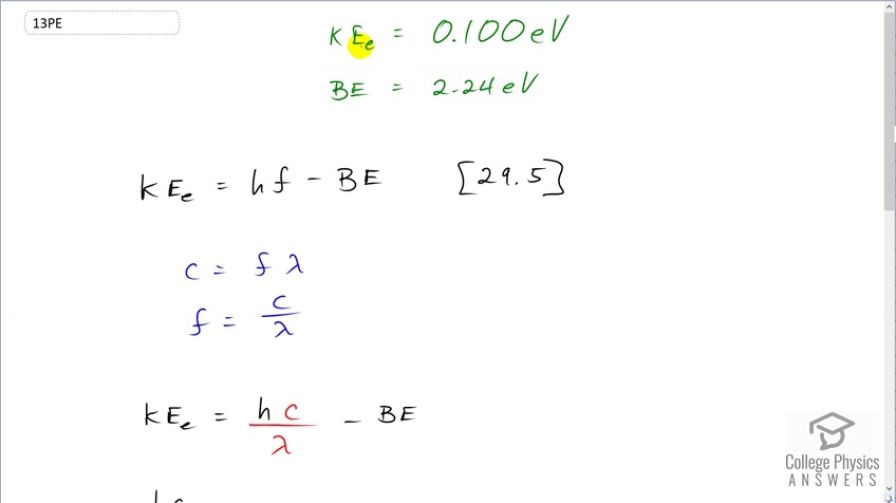
Find the wavelength of photons that eject 0.100-eV electrons from potassium, given that the binding energy is 2.24 eV. Are these photons visible?
Final Answer
Yes, these photons are visible as the color yellow-green.
Solution video
OpenStax College Physics for AP® Courses, Chapter 29, Problem 13 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. Potassium has an electron binding energy of 2.24 electron volts and we are told that electrons ejected by a certain wavelength of photon has a kinetic energy of 0.100 electron volts and our job is to figure out, what is the wavelength of the photons that caused these electrons to be ejected? So equation [29.5] tells us that the kinetic energy of the electron is Planck's constant times the frequency of the photon minus the binding energy of the material, in this case potassium. We'll substitute for f because we want to find λ, we want to find wavelength. So the speed of light is frequency times wavelength and we divide both sides by λ to solve for f and then we substitute for f with c over λ here. So we have to isolate this thing so we are gonna add binding energy to both sides and then switch the signs around and we get hc over λ equals kinetic energy plus binding energy. And then we are gonna multiply both sides by λ and divide both sides by this sum of kinetic energy plus binding energy. And then that gives us λ. It is Planck's constant times c over the kinetic energy plus the binding energy. So that's Planck's constant times the speed of light divided by 0.100 electron volts plus 2.24 electron volts giving us 531 nanometers. And we chose this unit for Planck's constant— electron volts— in order to match with the denominator energies, which are also in electron volts. And yes, these photons are visible; they are yellowish-green.
