Question
(a) Calculate the amount of microwave energy in joules needed to raise the temperature of 1.00 kg of soup from to . (b) What is the total momentum of all the
microwave photons it takes to do this? (c) Calculate the velocity of a 1.00-kg mass with the same momentum. (d) What is the kinetic energy of this mass?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 29, Problem 76 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
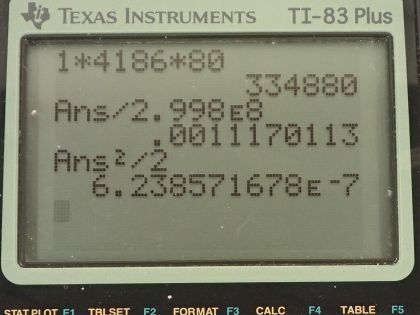
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. How much energy is needed to raise 1.00 kilogram of soup from a temperature of 20.0 degrees Celsius to 100 degrees Celsius? We need to know the change in temperature then is going to be the difference between its final temperature and its initial temperature so 100 minus 20.0 is going to be 80.0 Celsius degrees of temperature change. The total energy required will be this mass times the specific heat times the change in temperature and we'll assume that the specific heat of the soup is the same as that of water 4186 joules per kilogram per Celsius degree. So the energy required from the microwave photons then is going to be 1.00 kilogram of mass times 4186 joules per kilogram per Celsius degree times 80.0 Celsius degrees— temperature change— which is 3.35 times 10 to the 5 joules. What is the total momentum of all the microwave photons it takes to do this? Well the total momentum then is going to be the number of photons multiplied by the momentum of a single photon and the momentum of a single photon is the energy of that photon divided by the speed of light and we can write this then as the number of photons times the energy divided by speed of light and this numerator— the number of photons times the energy per photon— is the total energy of all the photons and that is Q up here. So the total momentum then is 3.3488 times 10 to the 5 joules divided by the speed of light and that is 1.12 times 10 to the minus 3 kilogram meters per second and then the velocity of a hypothetical 1.00 kilogram mass that has the same momentum well, momentum is mass times velocity and we can divide both sides by m to solve for v and so the velocity of this hypothetical 1.00 kilogram mass will then be 1.117 times 10 to the minus 3 kilogram meters per second—momentum— divided by a kilogram of mass and that is 1.12 times 10 to the minus 3 meters per second. And lastly what is the kinetic energy of this mass? Well one-half times its mass times its velocity squared and then I substituted momentum divided by mass in place of v which we can do here and that makes p squared over 2m is going to be our formula for kinetic energy. So this is the momentum calculated in part (b) and we square that and divide by 2 times the mass and that works out to 6.24 times 10 to the minus 7 joules.